Fig. 1.
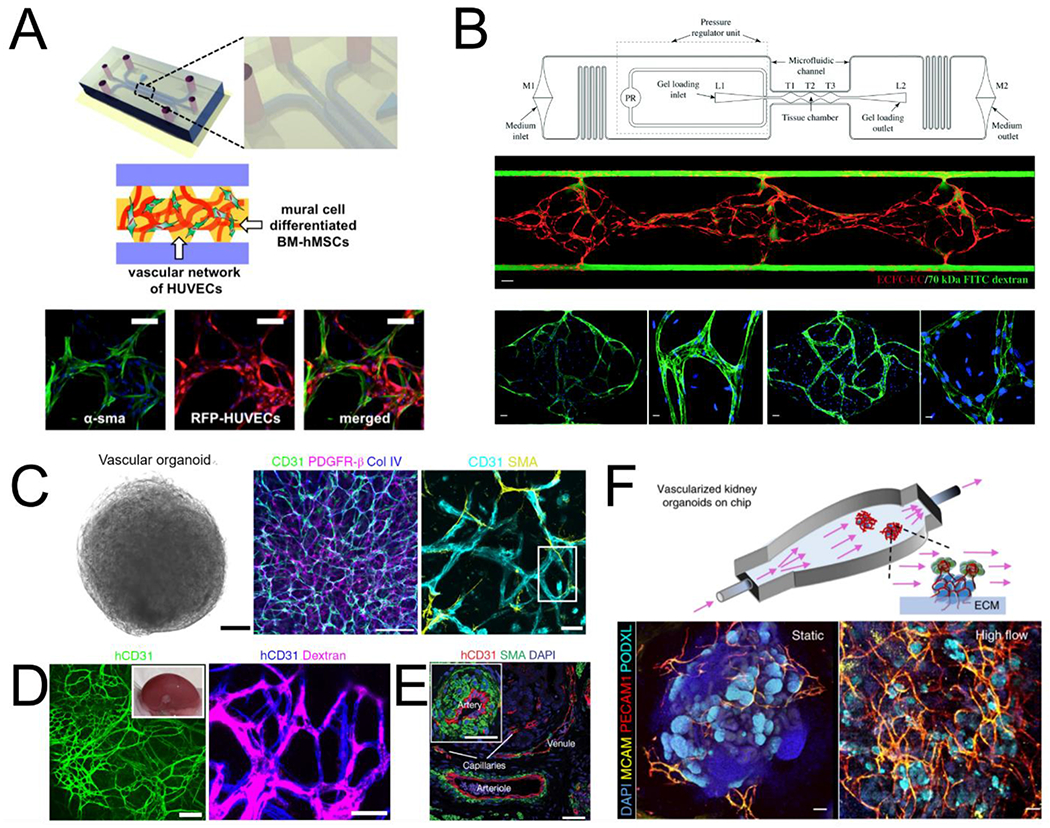
Models of vascular self-assembly. (A) Endothelial cells and mural cells embedded in a fibrin matrix and supported by perfusion self-assemble into perfusable networks with close contact between cell types (adapted from Jeon et al[49]). (B) Controlled interstitial flow and mass transport in a microfluidic design (top) across EC-laden hydrogels stimulates vasculogenesis and the formation of perfusable vascular networks (adapted from Phan et al[51]). Middle panel: three tissue chamber with red for ECs and green for perfusion of 70kDa FITC-Dextran. Bottom panels: staining of claudin-5 (left two), and VE-Cad (right two). (C) Vascular organoids after 15 days of differentiation compact into a sphere comprised of CD31 expressing endothelial cells that are enveloped with mural cells (PDGFR-β, SMA) and basement membrane (COL IV). (D) hVOs transplanted in mice survive and form robust connection with the host vasculature. (E) > 1 month after transplantation, hVOs develop hierarchical vascular structures identifiable as arteries, venules, and capillaries (C,D,E adapted from Wimmer et al[62]). (F) Using microfluidics to deliver flow through vascularized kidney organoids enhanced vascular network formation as well as maturation of kidney organoid parenchyma (adapted from Homan et al[68]).
