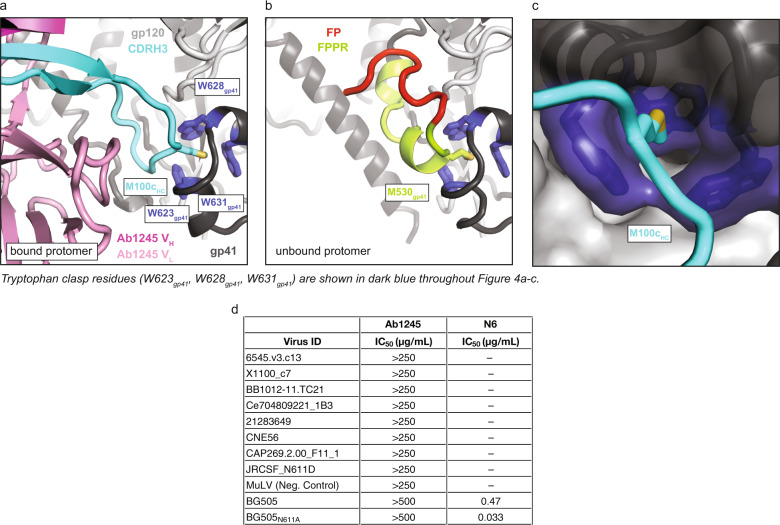
Fig. 4. Ab1245 CDRH3 mimics gp41 interactions with the tryptophan clasp.
a Cartoon representation of the interactions between Ab1245 VH-VL domains (pink with CDRH3 in cyan) and the tryptophan clasp of gp41 (gp41 in dark gray with Trp residues 623gp41, 628gp41, and 631gp41 in dark blue) with gp120 in light gray. Interacting residues between M100cHC and the tryptophan clasp shown as sticks. b Cartoon representation of the same view of an unbound protomer of gp41 with the portion of gp41 containing the fusion peptide (red) and fusion peptide proximal region (FPPR, green) interacting with the gp41 tryptophan clasp (same coloring as in (a)). Interactions between M530gp41 and the tryptophan clasp are shown as sticks. c Surface representation of gp41 (dark gray) and gp120 (light gray) with tryptophan clasp residues in dark blue. Ab1245 CDRH3 is cyan with a stick representation for the Met100cHC sidechain. d In vitro neutralization assays using IgGs Ab1245 or N666 (positive control for neutralization) against the indicated viral strains. In addition to those listed, Ab1245 was tested in-house against pseudoviruses from strains CE0217, CNE55, JRCSF, Du422, T250-4, Tro, X1632, 246F3, CH119, CE1176, BJOX002000_03_02, 25710, X2278, CNE8, and 398F1 at a top concentration of 500 µg/mL or 1000 µg/mL along with IgG N6 (positive control at a top concentration of 10 µg/mL). N6 was not evaluated in against the strains indicated by a dash because its neutralization potencies were previously published66. Whereas N6 exhibited expected neutralization potencies against evaluated strains66, Ab1245 exhibited no neutralization activity.