Abstract
Background
Tobacco smoking is associated with a reduced risk of developing sarcoidosis, and we previously reported that nicotine normalizes immune responses to environmental antigens in patients with active pulmonary sarcoidosis. The effects of nicotine on the progression of pulmonary sarcoidosis are unknown.
Research Question
Is nicotine treatment well tolerated, and will it improve lung function in patients with active pulmonary sarcoidosis?
Study Design and Methods
With local institutional review board approval, a randomized, double-blind, controlled pilot trial was conducted of daily nicotine transdermal patch treatment (21 mg daily) or placebo patch use for 24 weeks. The Ohio State University Wexner Medical Center and Cleveland Clinic enrolled 50 consecutive subjects aged ≥ 18 years with active pulmonary sarcoidosis, based on symptoms (ie, dyspnea, cough) and objective radiographic evidence of infiltrates consistent with nonfibrotic lung disease. Each study group was compared at 26 weeks based on repeated measures of FVC, FEV1, quantitative lung texture score based on CT texture analysis, Fatigue Assessment Score (FAS), St. George’s Respiratory Questionnaire (SGRQ), and the Sarcoidosis Assessment Tool.
Results
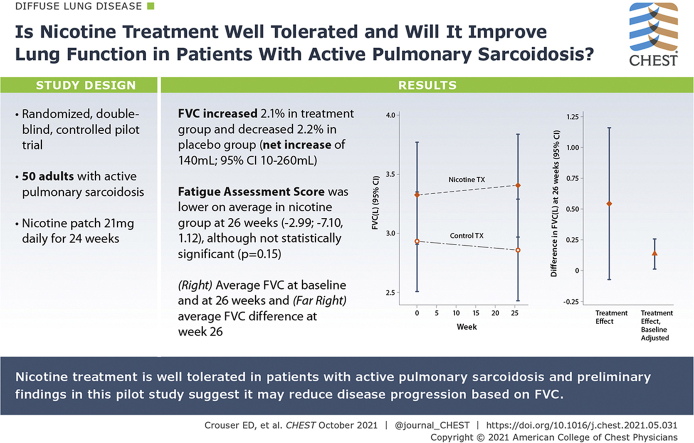
Nicotine treatment was associated with a clinically significant, approximately 2.1% (70 mL) improvement in FVC from baseline to 26 weeks. FVC decreased by a similar amount (2.2%) in the placebo group, with a net increase of 140 mL (95% CI, 10-260) when comparing nicotine vs placebo groups at 26 weeks. FEV1 and FAS improved marginally in the nicotine-treated group, compared with those on placebo. No improvement was observed in lung texture score, FAS, St. George’s Respiratory Questionnaire score, or the Sarcoidosis Assessment Tool. There were no reported serious adverse events or evidence of nicotine addiction.
Interpretation
Nicotine treatment was well tolerated in patients with active pulmonary sarcoidosis, and the preliminary findings of this pilot study suggest that it may reduce disease progression, based on FVC.
Clinical Trial Registration
ClinicalTrials.gov; No.: NCT02265874; URL: www.clinicaltrials.gov.
Key Words: fatigue, FVC, granuloma, human, interstitial lung disease, nicotinic receptor, randomized controlled trial
Abbreviations: fas, Fatigue Assessment Score; LTS, lung texture score
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 1169
Sarcoidosis is a granulomatous disease of unknown cause that most often involves the lungs but can affect virtually any other part of the body, and for which highly effective and well-tolerated therapies are lacking. A recent expert consensus statement recommends corticosteroids as the first-line therapy for sarcoidosis.1 However, patients with sarcoidosis often denounce the use of steroids based on common adverse side effects such as weight gain, mood swings, easy bruising, osteoporosis, glucose intolerance, and hypertension.2 Systemic glucocorticoids also increase the risk of serious infections.3 From a patient perspective, the studies indicate that patients with active sarcoidosis experience a dose-dependent reduction in perceived quality of life with increasing corticosteroid dose.4 Thus, the currently accepted front-line therapy for sarcoidosis is inadequate.
Compelling evidence indicates that nicotine can modulate an array of inflammatory conditions, including granulomatous diseases. Indirect evidence is provided by smokers who are chronically exposed to nicotine and are protected from hypersensitivity pneumonitis5 and sarcoidosis,6,7 and smoking increases the risk of spread of TB, an infectious disease that is regulated by granulomatous inflammation,8 presumably due to suppression of the immune response by nicotine.9 Direct evidence indicates that nicotine suppressed hypersensitivity pneumonitis in a mouse model,10 and preliminary data suggest that nicotine enemas may be beneficial for the treatment of Crohn’s disease.11 Nicotine is shown to normalize innate immune responses in patients with active pulmonary sarcoidosis.12 The mechanism by which nicotine suppresses granulomatous inflammation is unclear. However, studies indicate that activation of alpha-7 nicotinic receptors encourages autophagy,13 which could promote antigen clearance, and polarizes macrophages toward a regulatory phenotype.14
Based on the compelling evidence indicating that nicotine suppresses granulomatous inflammation, we hypothesized that sustained nicotine treatment would attenuate the progression of pulmonary sarcoidosis. We further hypothesized that nicotine would be well tolerated and safe in patients with active pulmonary sarcoidosis, which would be of critical importance and interest to patients given the current conventional treatment options. To this end, we conducted a randomized, double-blinded, placebo-controlled pilot study of nicotine patches for the treatment of active pulmonary sarcoidosis aimed at providing preliminary estimates of efficacy, along with critical information on side effects and patient acceptance.15
Patients and Methods
With single institutional review board approval, led by OSU, we conducted a randomized, double-blind, controlled pilot study of daily nicotine transdermal patch treatment and placebo at 2 sites: The Ohio State University Wexner Medical Center and the Cleveland Clinic. Additional details regarding the clinical trial design, recruitment, and intervention and methods were recently published.15 Subjects aged ≥ 18 years were enrolled who fulfilled the current American Thoracic Society criteria for sarcoidosis,16 with evidence of active pulmonary sarcoidosis, based on symptoms (ie, dyspnea, cough), biopsy-confirmed noncaseating granulomas (and no evidence of alternative diagnosis), and objective radiographic evidence of infiltrates consistent with nonfibrotic lung disease, as per the physician investigators in collaboration with expert thoracic radiologists. Importantly, patients were excluded who received doses of corticosteroids exceeding 10 mg/d or equivalent or steroid-sparing agents, such as methotrexate and azathioprine, or anti-tumor necrosis factor antibody treatments. Also excluded were those exposed to nicotine within 1 month of enrollment and those considered at some risk for complications related to the sympathomimetic properties of nicotine, including active neurologic or cardiovascular disease or subjects with active malignancy within 1 year of study enrollment.
Study Procedures
Eligible and consented patients were randomized, through a Web-based centralized system, stratified according to site and with equal allocation, to receive transdermal nicotine therapy or an identical placebo control patch.15 To reduce side effects, the nicotine patch dose was gradually increased at 1-week intervals from 7 mg to 14 mg to 21 mg daily, as tolerated. Those who could not tolerate dose escalation were maintained on their highest tolerated dose (up to 21 mg daily) for 24 weeks. To avoid withdrawal symptoms at study termination, the nicotine dose was tapered by 7 mg at weekly intervals prior to discontinuation. Study treatment was distributed on the day of randomization and at the week 10 visit, or as needed due to dose de-escalation. Patient-reported use and serum cotinine, a nicotine metabolite, was used to monitor compliance with allocated treatment at study weeks 10 and 26. Participants, as well as study coordinators, treating physicians, and the primary study statistician, were blinded as to the randomized treatment received.
Primary and Secondary End Points
Although the study was not powered to establish efficacy, it was designed to provide preliminary estimates of the difference between randomized groups for FVC change, the primary end point, at study week 26 (following 24 weeks of treatment), as well as an estimate of the difference in the change from baseline to study week 26 between randomized groups in FVC. FVC and FEV1 were measured from pulmonary function tests and were corrected for race by using the National Health and Nutrition Examination Survey equation.17 An additional outcome was to measure if sustained nicotine patch treatments were well tolerated by patients with active pulmonary sarcoidosis, quantified through reports of side effects, adverse events, and continued use of the patches.
Objective changes in lung manifestations of sarcoidosis were assessed from a computer-generated lung texture score (LTS) derived from lung CT scans at baseline and study week 26. LTS is designed to disregard normal anatomical features (eg, linear structures such as blood vessels, airways, lymphatics) to emphasize the signal from diseased tissue (eg, nodular, fibrotic manifestations), as described in full detail in our previous publication.18 Standardized surveys were administered to assess symptoms of fatigue (Fatigue Assessment Scale)19 and sarcoidosis-related changes in quality of life (Sarcoidosis Assessment Tool),20 and dyspnea (St. George’s Respiratory Questionnaire) at baseline and study weeks 10, 18, and 26.21 To screen for nicotine addiction, we contacted all study subjects approximately 1 month following completion of the study to determine if they were using nicotine-containing products.
Statistical Methods
Study sample size and design considerations have been previously detailed.15 Our planned study recruitment goal was to randomize 25 participants per treatment group, for a total of 50 participants. We expected that this number of patients would provide robust data for planning a subsequent Phase III trial but would not have high power to detect the expected improvements in those randomized to receive nicotine therapy, expected to be an effect around 3%. Participant demographic, medical history, and clinical baseline characteristics are described according to randomized group and overall to characterize the cohort. Primary and secondary analyses were conducted by randomized group in line with an intention-to-treat model. Longitudinal linear mixed effects models were used to estimate the average difference as well as the average differences in the change from baseline between randomized groups, while accounting for measurements within participants over time. All three repeated measures at baseline and week 26 of FVC and FEV were included in these models. Longitudinal models include fixed effects for randomized treatment group, time point (baseline, week 10, week 18, and week 26 where available), and the interaction between treatment group and time, as well as a random intercept for each participant and unstructured covariance structure. A priori, adjustment for sex was considered potentially important as a prognostic factor for study outcomes and included in longitudinal models.
CIs and P values are all two-sided and presented at the nominal level. Data management was conducted by using SAS version 9.4 (SAS Institute, Inc.). Analysis was performed in Stata release 15 (StataCorp LLC).
Results
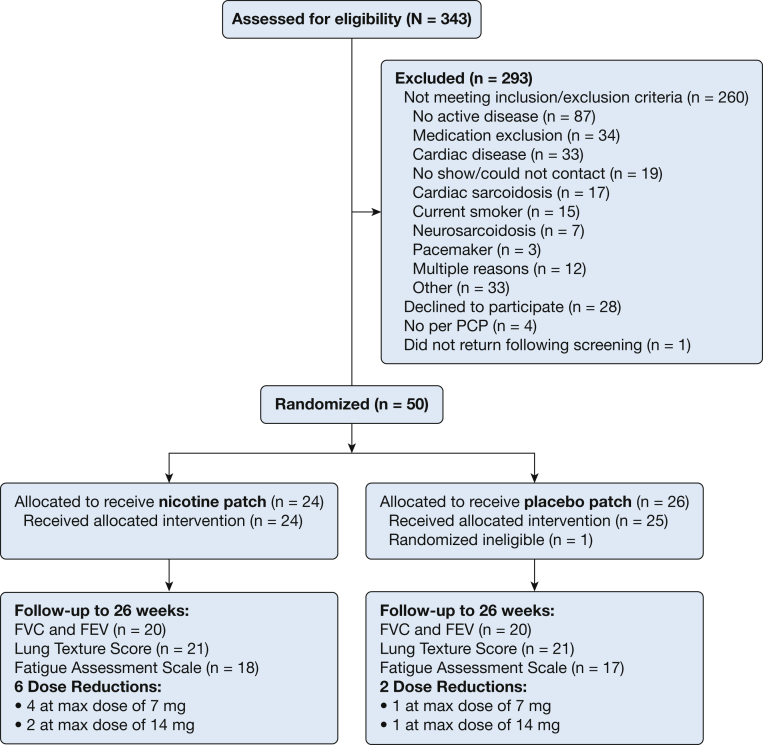
Between October 2015 and January 2019, fifty participants were randomized to study treatment. Following randomization, one participant was determined not to have active disease, did not receive any study therapy, and was excluded from follow-up, leaving 49 randomized and eligible participants. Of these 49, a total of 40 (81.6%) participants were followed up through the end of study week 26 (Fig 1). Three participants withdrew from study treatment; one was unable to comply with study treatment and follow-up (randomized to the placebo group), one chose to discontinue treatment due to side effects at study week 3 (randomized to the nicotine group; itching at patch site), and one discontinued due to adverse side effects that included nausea, vivid dreams, and vomiting at study week 13 (randomized to the nicotine group). Of the remaining six participants who were lost to follow-up, three discontinued prior to study week 10. Eight participants had a reduced (or no increase) dose of their randomized treatment due to reported side effects such as nausea, dizziness, and headache, six of whom were randomized to receive nicotine. As evidenced by serum cotinine levels, two participants randomized to receive placebo had detectable nicotine levels, whereas six of those randomized to receive nicotine treatment did not have detectable levels at study week 26. No serious adverse events attributable to study treatment were observed, and none of the patients who were randomized to nicotine treatment reported continued use of nicotine products approximately 1 month following study completion.
Figure 1.
Study recruitment and follow-up. max = maximum; PCP = primary care physician.
Generally, baseline characteristics of patients receiving the nicotine patch and placebo did not clinically differ. Overall, the majority of participants had Medical Research Council Dyspnea grade 1 (56%), were female (61%), and were on average 54 (±SD 10) years of age at enrollment. Nine subjects in the nicotine patch group (37.5%) and seven in the control group (28.0%) were taking low-dose prednisone (≤ 10 mg/d) for at least 1 month at the time of enrollment, with evidence of active disease despite this treatment. The prednisone dose was not adjusted during the duration of the study. Despite randomization, those randomized to the nicotine treatment group by chance had clinically higher baseline FVC than those in the placebo group (Table 1), which exceeded 5%, a clinically significant difference relative to reported minimal clinically important FVC change in interstitial lung disease trials.22 Of those randomized to receive the nicotine patch, 38% were new to treatment, and 24% were in the placebo patch group.
Table 1.
Baseline Characteristics According to Randomized Group
| Characteristic | Nicotine Patch (n = 24) | Placebo Patch (n = 25) | Total (N = 49) |
|---|---|---|---|
| Recruitment site | |||
| Cleveland Clinic | 7 (29.2) | 9 (36.0) | 16 (32.7) |
| The Ohio State University | 17 (70.8) | 16 (64.0) | 33 (67.3) |
| Race and ethnicitya | |||
| White non-Hispanic | 15 (65.2) | 13 (56.5) | 28 (60.9) |
| Black non-Hispanic | 6 (26.1) | 10 (43.5) | 16 (34.8) |
| Hispanic | 2 (8.7) | 0 | 2 (4.3) |
| Sex | |||
| Female | 13 (54.2) | 17 (68.0) | 30 (61.2) |
| Male | 11 (45.8) | 8 (32.0) | 19 (38.8) |
| Baseline BMI categoryb | |||
| Underweight/normal | 3 (12.5) | 4 (16.0) | 7 (14.2) |
| Overweight | 9 (37.5) | 8 (36.4) | 17 (37.0) |
| Obese | 12 (50.0) | 10 (40.0) | 22 (44.8) |
| Smoking status | |||
| Former smoker | 7 (29.2) | 2 (8.0) | 9 (18.4) |
| Never smoker | 17 (70.8) | 23 (92.0) | 40 (81.6) |
| Medical Research Council grade | |||
| 1: Shortness of breath with minimal exertion | 12 (52.2) | 15 (60.0) | 27 (56.3) |
| 2: Walks slower than people of the same age on the level because of breathlessness | 9 (39.1) | 6 (24.0) | 15 (31.3) |
| 3: Stops for breath after walking about 100 m | 2 (8.7) | 2 (8.0) | 4 (8.3) |
| 4: Breathless while performing activities of daily living | 0 | 2 (8.0) | 2 (4.2) |
| Treatment history | |||
| Newly treated, acute | 9 (37.5) | 6 (24.0) | 15 (30.6) |
| Chronically active | 15 (62.5) | 19 (76.0) | 34 (69.4) |
| Medical history (affected body systems) | |||
| Respiratory | 22 (91.7) | 22 (88.0) | 44 (89.8) |
| Cardiovascular | 14 (58.3) | 13 (52.0) | 27 (55.1) |
| Endocrine/metabolic | 11 (45.8) | 12 (48.0) | 23 (46.9) |
| HEENT | 9 (37.5) | 11 (44.0) | 20 (40.8) |
| GI | 9 (37.5) | 11 (44.0) | 20 (40.8) |
| Musculoskeletal | 10 (41.7) | 8 (32.0) | 18 (36.7) |
| Genitourinary/reproductive | 7 (29.2) | 8 (32.0) | 15 (30.6) |
| Allergy | 5 (20.8) | 8 (32.0) | 13 (26.5) |
| Hematologic/lymphatic | 7 (29.2) | 5 (20.0) | 12 (24.5) |
| Neurologic/CNS | 4 (16.7) | 6 (24.0) | 10 (20.4) |
| Psychological/psychiatric | 4 (16.7) | 5 (20.0) | 9 (18.4) |
| Dermatological | 4 (16.7) | 5 (20.0) | 9 (18.4) |
| Hepatic | 3 (12.5) | 2 (8.0) | 5 (10.2) |
| Other | 6 (25.0) | 8 (32.0) | 14 (28.6) |
| Symptoms | |||
| Fatigue | 13 (54.2) | 8 (32.0) | 21 (42.9) |
| Dyspnea | 18 (75.0) | 20 (80.0) | 38 (77.6) |
| Cough | 10 (41.7) | 8 (32.0) | 18 (36.7) |
| Chest pain | 2 (8.3) | 2 (8.0) | 4 (8.2) |
| Skin rash | 1 (4.2) | 2 (9.0) | 3 (6.1) |
| Arthralgia | 1 (4.2) | 1 (4.0) | 2 (4.1) |
| Swollen lymph nodes | 1 (4.2) | 0 | 1 (2.0) |
| Headache | 1 (4.2) | 0 | 1 (2.0) |
| Weakness | 0 | 1 (4.0) | 1 (2.0) |
| Other | 12 (50.0) | 13 (52.0) | 25 (51.0) |
| Age at enrollment, y | 51.8 ± 11.5 | 55.8 ± 8.0 | 53.9 ± 10.0 |
| Baseline BMI, kg/m2b | 30.9 ± 8.2 | 23.8 ± 7.0 | 31.9 ± 7.6 |
| Baseline FVC, L | 3.3 ± 1.1 | 2.9 ± 1.1 | 3.1 ± 1.1 |
| Baseline % predicted FVCc | 86.0 ± 18.8 | 78.7 ± 17.3 | 82.2 ± 18.2 |
| Baseline FEV1 | 2.5 ± 0.7 | 2.2 ± 0.9 | 2.3± 0.8 |
| Baseline % predicted FEV1 | 77.7 ± 15.2 | 73.6 ± 21.1 | 75.6 ± 18.4 |
| Baseline FEV/FVCd | 73.6 ± 7.3 | 74.9 ± 11.5 | 74.3 ± 9.6 |
| Pack years of tobacco cigarette smokinge | 15 (1-24) | 13 (5-21) | 15 (4-21) |
Data are expressed as No. (%) or mean ± SD unless otherwise indicated. HEENT = head, eyes, ears, nose, and throat.
Three participants missing race/ethnicity.
Three participants missing BMI.
Based on National Health and Nutrition Examination Survey criteria.
One participant missing FEV/FVC.
Among former smokers at baseline (n = 9 [nicotine group, n = 7; placebo group, n = 2]); median and interquartile range.
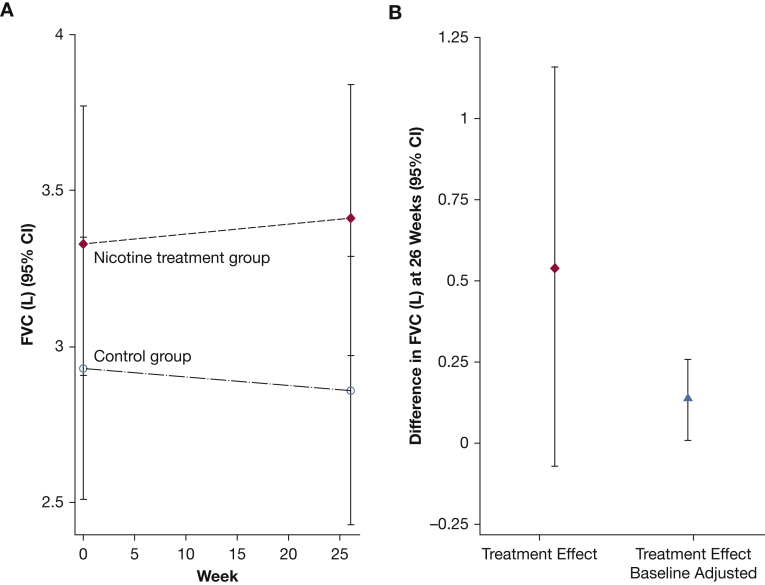
During the 24-week study period, mean FVC decreased by 2.4% (70 mL) in the placebo group and increased by 2.1% (70 mL) in the nicotine group, for a mean treatment effect of 140 mL (95% CI, 10-260) (Fig 2, Table 217, 18, 19, 20). Similarly, greater lung disease burden, reflected by higher LTS, was observed in the placebo group (mean ± SE, 66.2 ± 4.6) compared with the nicotine treatment group (mean ± SE, 61.4 ± 4.7). There was no evidence to suggest that LTS differed between treatment groups following 24 weeks of treatment, or that the difference in the change from baseline was clinically different between randomized groups.
Figure 2.
A, Average FVC (95% CI) according to randomized treatment group at baseline and study week 26. B, Average FVC (95% CI) difference at week 26.
Table 2.
Primary and Secondary Outcomes According to Randomized Group
| Outcome | Nicotine Patch n = 24 Mean ± SE [n] |
Placebo Patch n = 25 Mean ± SE [n] |
Difference (95% CI), P Value | Sex-Adjusted Difference (95% CI), P Value |
|---|---|---|---|---|
| FVC, La | ||||
| Week 26 | 3.40 ± 0.223 [n = 20] | 2.86 ± 0.219 [n = 20] | 0.54 (–0.07 to 1.16) .081 | 0.36 (–0.14 to 0.87) .158 |
| Baseline | 3.34 ± 0.220 [n = 24] | 2.93 ± 0.215 [n = 25] | ||
| Change from baseline | 0.07 ± 0.04 | –0.07 ± 0.04 | 0.14 (0.01 to 0.26) .015 | 0.14 (0.03 to 0.25) .015 |
| FEV1a | ||||
| Week 26 | 2.51 ± 0.167) [n = 20] | 2.20 ± 0.164 [n = 20] | 0.31 (–0.15 to 0.77) .190 | 0.18 (–0.21 to 0.56) .372 |
| Baseline | 2.47 ± 0.167 [n = 24] | 2.21 ± 0.164 [n = 25] | ||
| Change from baseline | 0.037 ± 0.020 | –0.0081 ± 0.020 | 0.05 (–0.01 to 0.10) .120 | 0.05 (–0.002 to 0.09) .061 |
| Lung texture score17a | ||||
| Week 26 | 60.38 ± 4.70 [n = 21] | 68.74 ± 4.65 [n = 21] | –8.36 (–21.32 to 4.61) .206 | –8.11 (–21.30 to 5.07) .228 |
| Baseline | 61.35 ± 4.72 [n = 24] | 66.19 ± 4.62 [n = 25] | ||
| Change from baseline | –0.97 ± 3.46 | 2.54 ± 3.45 | –3.51 (–13.10 to 6.07) .473 | –3.50 (–13.09 to 6.08) .474 |
| Fatigue Assessment Scale18ab | ||||
| Week 26 | 23.60 ± 1.55 [n = 18] | 25.86 ± 1.58 [n = 17] | –2.26 (–6.61 to 2.08) .307 | –1.66 (–5.64 to 2.32) .414 |
| Baseline | 26.42 ± 1.37 [n = 23] | 25.69 ± 1.37 [n = 23] | ||
| Change from baseline | –2.82 ± 1.46 | 0.171 ± 1.50 | –2.99 (–7.10 to 1.12) .154 | –2.93 (–7.02 to 1.17) .161 |
| Sarcoidosis Assessment Tool19ab | ||||
| Week 26 | 116.17 ± 4.82 [n = 14] | 118.30 ± 4.51 [n = 17] | –2.13 (–15.07 to 10.81) .757 | –1.85 (–13.93 to 10.23) .764 |
| Baseline | 121.92 ± 4.05 [n = 19] | 123.64 ± 3.90 [n = 21] | ||
| Change from baseline | –5.75 ± 3.91 | –5.34 ± 3.42 | –0.415 (–10.60 to 9.77) .936 | –0.91 (11.16 to 9.34) .862 |
| St. George’s Respiratory Questionnaire20a | ||||
| Week 26 | 35.15 ± 4.76 [n = 18] | 34.21 ± 4.52 [n = 19] | 0.94 (–11.93 to 13.82) .886 | 3.42 (–9.33 to 16.16) .599 |
| Baseline | 40.72 ± 4.14 [n = 21] | 40.99 ± 3.88 [n = 24] | ||
| Change from baseline | –5.57 ± 2.84 | –6.78 ± 2.76 | 1.21 (–6.56 to 8.97) .761 | 1.09 (–6.71 to 8.88) .785 |
| Serum cotininea | ||||
| Week 26 | 136.64 ± 28.31 [n = 19] | 23.84 ± 28.03 [n = 19] | 112.80 (34.71 to 190.90) .005 | 111.44 (32.07 to 190.81) .006 |
| Baseline | 39.92 ± 20.70 [n = 24] | 33.68 ± 20.28 [n = 25] | ||
| Change from baseline | 96.72 ± 20.26 | –9.84 ± 20.26 | 106.56 (50.40 to 162.72) < .001 | 106.62 (50.44 to 162.79) < .001 |
Estimates obtained from a linear mixed effects model that accounted for correlation within participants over time, and when applicable in repeated measurements at each time point (FVC). Longitudinal models included an indicator variable for randomized treatment and a categorical variable for time point as main effects, as well as their interaction term.
Only participants with all non-missing scale components were included.
Of the patient-reported surveys, the most clinically meaningful difference between treatment groups was observed in the Fatigue Assessment Score (FAS) (difference in the change from baseline, –2.99; 95% CI, –7.10 to 1.12), with those randomized to receive nicotine treatment having lower, on average, FAS at 26 weeks. There was no evidence to suggest that the SAT or St. George’s Respiratory Questionnaire scale measures at study week 26, or their change from baseline, differed meaningfully between randomized groups (Table 2).
Interpretation
Based on a body of evidence indicating that smoking nicotine-containing cigarettes is protective against developing sarcoidosis,6,7 and showing nicotine to be immune modulating,9, 10, 11, 12, 13, 14 we report herein on the first randomized controlled pilot study to determine if nicotine is well tolerated and is potentially beneficial for the treatment of sarcoidosis. The study was underpowered to show the expected treatment effect for nicotine treatment, and despite randomization, there were large baseline differences in FVC. Following adjustment for baseline FVC,23 nicotine treatment was associated with a modest but statistically significant improvement in FVC with a similar reduction in FVC in the control group. Moreover, nicotine was well tolerated and was not addictive.
FVC is considered to be the most validated clinical end point for interstitial lung diseases, including autoimmune lung disease,24 fibrotic lung disease,22 and in sarcoidosis,25,26 and this pilot study showed a statistically significant change in FVC in the nicotine treatment group. However, despite randomization, the observed differences in FVC results in the nicotine and control groups were influenced by large baseline FVC variability in the treatment arms. As such, direct comparison of FVC following 24 weeks of treatment between randomized groups was challenging. However, the difference in FVC adjusted for baseline (a net change of 140 mL) is encouraging.
In contrast to other studies designed to assess third-line therapies for patients with otherwise treatment-refractory pulmonary sarcoidosis, which showed much smaller improvements in FVC,27 the intended use of nicotine in our trial is as a first- or second-line treatment (eg, instead of steroids or to reduce reliance on steroids). Thus, our pilot nicotine trial is more comparable to those using corticosteroids as first-line treatment in which larger (7%-10%) FVC improvements were documented during the initial phases of treatment.28, 29, 30 Several studies have shown that the quality of life is reduced on a dose-dependent basis among patients with pulmonary sarcoidosis who are treated with corticosteroids.4,31 A recent meta-analysis indicated that chronic corticosteroid exposure among patients with immune-mediated inflammatory diseases, even at low doses, is associated with a significant risk of cardiovascular complications.32 Thus, our preliminary findings suggest that nicotine could serve as a well-tolerated alternative to corticosteroids, the currently recommended first-line treatment for sarcoidosis.1
Preservation of lung function, as reflected by FVC, is a limited surrogate for sarcoidosis disease burden because nonpulmonary manifestations may have important implications for overall quality of life. For instance, deconditioning is a common complication of sarcoidosis, and exercise has been shown to improve effort-dependent metrics such as FVC and 6-min walk distance, presumably due to improved conditioning.33 Fatigue and inability to focus or process new information are very common symptoms among patients with active pulmonary sarcoidosis, and they have important implications for lower quality of life.34 Activation of nicotinic receptors in the brain results in CNS activation that is shown to improve cognitive function and suppresses appetite.35 The results of this small pilot study provide preliminary evidence suggesting an encouraging trend toward improvement in fatigue symptoms based on the FAS, a metric that has been validated for use in patients with sarcoidosis.19
There are a number of limitations of this pilot study that were largely influenced by the small study size. Due to the limited study size, the observed statistically significant changes in FVC between groups following 24 weeks of treatment and within groups compared with baseline should be considered preliminary supportive evidence and should not encourage health care providers to treat patients with nicotine patches. Likewise, nonstatistical trends for FAS and other quality of life measures, as well as LTS derived from CT scans, should be viewed as inconclusive pending a larger clinical trial. Finally, the small size of the study does not allow us to state with certainty that transdermal nicotine patch treatment is not addictive. However, a prior study of similar design, except of 12 weeks’ duration instead of 24 weeks, also reported no evidence of nicotine addiction.12
There are a number of potential advantages to further evaluating nicotine as a new, front-line therapy for sarcoidosis. As opposed to corticosteroids (the primary treatment used for sarcoidosis), nicotine does not promote weight gain, glucose intolerance, or susceptibility to severe infections or cardiovascular disease,32 and it has the added benefit of potentially reducing fatigue. Nicotine has advantages over steroid-sparing agents, such as methotrexate and azathioprine, including lower risk for hepatotoxicity and bone marrow suppression and related infections. Furthermore, transdermal nicotine was shown to be well tolerated and safe, and is already approved by the US Food and Drug Administration for use in other human conditions.
Our preliminary findings in this pilot study indicate that nicotine patches are nonaddictive, but this possibility remains a concern. If shown to be safe and beneficial in a future, appropriately powered study, nicotine is poised to be rapidly repurposed for the treatment of sarcoidosis. Additional studies are required to determine if nicotine is a viable alternative to prednisone, the current recommended first-line therapy,1 for the treatment of pulmonary sarcoidosis.
Take-home Points.
Study Question: Is nicotine treatment well tolerated, and will it improve lung function in patients with active pulmonary sarcoidosis?
Results: Treatment with transdermal nicotine for 6 months was shown to be safe and was associated with a statistically significant improvement in lung function, as reflected by FVC. There was a trend toward improvement in fatigue scores in the nicotine treatment group.
Interpretation: This pilot study provides encouraging preliminary data in support of conducting additional research to determine if transdermal nicotine is efficacious for the treatment of pulmonary sarcoidosis.
Acknowledgments
Author contributions: E. D. C. is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article. Further details of author contributions are as follows: conception and design, E. D. C., D. A. C., and E. M. H.; performing experiments, E. D. C., M. W. J., K. M., J. B., C. D., and B. S. E.; analysis and interpretation, E. D. C, D. A. C., M. W. J., R. M. S., K. M., J. B., C. D., B. S. E., and E. M. H.; and drafting the manuscript for important intellectual content, E. D. C., R. M. S, D. A. C., M. W. J., C. D., B. S. E., and E. M. H.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors acknowledge the contributions of Data and Safety Monitoring Board members who provided guidance and assured data quality: Marc Judson, MD (Chair); Kenneth Knox, MD; Lora Reineck, MD; Lai Wei, PhD; Daren Knoell, PharmD; and Michael Para, MD. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
FUNDING/SUPPORT: This study was supported by the National Institutes of Health [Grant R34HL123586] and by the National Center for Advancing Translational Sciences [award number UL1TR001070].
References
- 1.Rahaghi F.F., Baughman R.P., Saketkoo L.A. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29(155):190146. doi: 10.1183/16000617.0146-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Judson M.A. Developing better drugs for pulmonary sarcoidosis: determining indications for treatment and endpoints to assess therapy based on patient and clinician concerns. F1000Res. 2019;8 doi: 10.12688/f1000research.20696.1. F1000 Faculty Rev-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossides M., Kullberg S., Eklund A. Risk of first and recurrent serious infection in sarcoidosis: a Swedish register-based cohort study. Eur Respir J. 2020;56(3):2000767. doi: 10.1183/13993003.00767-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Judson M.A., Chaudhry H., Louis A., Lee K., Yucel R. The effect of corticosteroids on quality of life in a sarcoidosis clinic: the results of a propensity analysis. Respir Med. 2015;109(4):526–531. doi: 10.1016/j.rmed.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cormier Y., Israel-Assayag E., Bedard G., Duchaine C. Hypersensitivity pneumonitis in peat moss processing plant workers. Am J Respir Crit Care Med. 1998;158(2):412–417. doi: 10.1164/ajrccm.158.2.9712095. [DOI] [PubMed] [Google Scholar]
- 6.Newman L.S., Rose C.S., Bresnitz E.A. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170(12):1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 7.Valeyre D., Soler P., Clerici C. Smoking and pulmonary sarcoidosis: effect of cigarette smoking on prevalence, clinical manifestations, alveolitis, and evolution of the disease. Thorax. 1988;43(7):516–524. doi: 10.1136/thx.43.7.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara G., Murray M., Winthrop K. Risk factors associated with pulmonary tuberculosis: smoking, diabetes and anti-TNFalpha drugs. Curr Opin Pulm Med. 2012;18(3):233–240. doi: 10.1097/MCP.0b013e328351f9d6. [DOI] [PubMed] [Google Scholar]
- 9.Valdez-Miramontes C.E., Trejo Martinez L.A., Torres-Juarez F. Nicotine modulates molecules of the innate immune response in epithelial cells and macrophages during infection with M. tuberculosis. Clin Exp Immunol. 2020;199(2):230–243. doi: 10.1111/cei.13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanchet M.R., Israel-Assayag E., Cormier Y. Inhibitory effect of nicotine on experimental hypersensitivity pneumonitis in vivo and in vitro. Am J Respir Crit Care Med. 2004;169(8):903–909. doi: 10.1164/rccm.200210-1154OC. [DOI] [PubMed] [Google Scholar]
- 11.Ingram J.R., Rhodes J., Evans B.K., Thomas G.A. Nicotine enemas for active Crohn's colitis: an open pilot study. Gastroenterol Res Pract. 2008;2008:237185. doi: 10.1155/2008/237185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julian M.W., Shao G., Schlesinger L.S. Nicotine treatment improves Toll-like receptor 2 and Toll-like receptor 9 responsiveness in active pulmonary sarcoidosis. Chest. 2013;143(2):461–470. doi: 10.1378/chest.12-0383. [DOI] [PubMed] [Google Scholar]
- 13.Shao B.Z., Wang S.L., Fang J., Li Z.S., Bai Y., Wu K. Alpha7 nicotinic acetylcholine receptor alleviates inflammatory bowel disease through induction of AMPK-mTOR-p70S6K-mediated autophagy. Inflammation. 2019;42(5):1666–1679. doi: 10.1007/s10753-019-01027-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q., Lu Y., Bian H., Guo L., Zhu H. Activation of the alpha7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. Am J Transl Res. 2017;9(3):971–985. [PMC free article] [PubMed] [Google Scholar]
- 15.Hade E.M., Smith R.M., Culver D.A., Crouser E.D. Design, rationale, and baseline characteristics of a pilot randomized clinical trial of nicotine treatment for pulmonary sarcoidosis. Contemp Clin Trials Commun. 2020;20:100669. doi: 10.1016/j.conctc.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crouser E.D., Maier L.A., Wilson K.C. Diagnosis and detection of sarcoidosis: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(8):e26–e51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crapo R.O., Morris A.H., Gardner R.M. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123(6):659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 18.Erdal B.S., Crouser E.D., Yildiz V. Quantitative computerized two-point correlation analysis of lung CT scans correlates with pulmonary function in pulmonary sarcoidosis. Chest. 2012;142(6):1589–1597. doi: 10.1378/chest.11-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michielsen H.J., De Vries J., Van Heck G.L. Psychometric qualities of a brief self-rated fatigue measure: the Fatigue Assessment Scale. J Psychosom Res. 2003;54(4):345–352. doi: 10.1016/s0022-3999(02)00392-6. [DOI] [PubMed] [Google Scholar]
- 20.Judson M.A., Mack M., Beaumont J.L., Watt R., Barnathan E.S., Victorson D.E. Validation and important differences for the Sarcoidosis Assessment Tool. A new patient-reported outcome measure. Am J Respir Crit Care Med. 2015;191(7):786–795. doi: 10.1164/rccm.201410-1785OC. [DOI] [PubMed] [Google Scholar]
- 21.Barr J.T., Schumacher G.E., Freeman S., LeMoine M., Bakst A.W., Jones P.W. American translation, modification, and validation of the St. George’s Respiratory Questionnaire. Clin Ther. 2000;22(9):1121–1145. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 22.du Bois R.M., Weycker D., Albera C. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. 2011;184(12):1382–1389. doi: 10.1164/rccm.201105-0840OC. [DOI] [PubMed] [Google Scholar]
- 23.Altman D.G., Dore C.J. Randomisation and baseline comparison in clinical trials. Lancet. 1990;335:149–153. doi: 10.1016/0140-6736(90)90014-v. [DOI] [PubMed] [Google Scholar]
- 24.Kafaja S., Clements P.J., Wilhalme H. Reliability and minimal clinically important differences of forced vital capacity: results from the Scleroderma Lung Studies (SLS-I and SLS-II) Am J Respir Crit Care Med. 2018;197(5):644–652. doi: 10.1164/rccm.201709-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeager H., Rossman M.D., Baughman R.P. Pulmonary and psychosocial findings at enrollment in the ACCESS study. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(2):147–153. [PubMed] [Google Scholar]
- 26.Baughman R.P., Shipley R., Desai S. Changes in chest roentgenogram of sarcoidosis patients during a clinical trial of infliximab therapy: comparison of different methods of evaluation. Chest. 2009;136(2):526–535. doi: 10.1378/chest.08-1876. [DOI] [PubMed] [Google Scholar]
- 27.Baughman R.P., Drent M., Kavuru M. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 28.Broos C.E., Poell L.H.C., Looman C.W.N. No evidence found for an association between prednisone dose and FVC change in newly-treated pulmonary sarcoidosis. Respir Med. 2018;138S:S31–S37. doi: 10.1016/j.rmed.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Spratling L., Tenholder M.F., Underwood G.H., Feaster B.L., Requa R.K. Daily vs alternate day prednisone therapy for stage II sarcoidosis. Chest. 1985;88(5):687–690. doi: 10.1378/chest.88.5.687. [DOI] [PubMed] [Google Scholar]
- 30.Pietinalho A., Tukiainen P., Haahtela T., Persson T., Selroos O., Finnish Pulmonary Sarcoidosis Study Group Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121(1):24–31. doi: 10.1378/chest.121.1.24. [DOI] [PubMed] [Google Scholar]
- 31.Cox C.E., Donohue J.F., Brown C.D., Kataria Y.P., Judson M.A. Health-related quality of life of persons with sarcoidosis. Chest. 2004;125(3):997–1004. doi: 10.1378/chest.125.3.997. [DOI] [PubMed] [Google Scholar]
- 32.Pujades-Rodriguez M., Morgan A.W., Cubban R.M., Wu J. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PLoS Med. 2020;17(12) doi: 10.1371/journal.pmed.1003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strookappe B., Swigris J., De Vries J., Elfferich M., Knevel T., Drent M. Benefits of physical training in sarcoidosis. Lung. 2015;193(5):701–708. doi: 10.1007/s00408-015-9784-9. [DOI] [PubMed] [Google Scholar]
- 34.Thunold R.F., Lokke A., Cohen A.L., Ole H., Bendstrup E. Patient reported outcome measures (PROMs) in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34(1):2–17. doi: 10.36141/svdld.v34i1.5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang H., Xu Y., van den Pol A.N. Nicotine excites hypothalamic arcuate anorexigenic proopiomelanocortin neurons and orexigenic neuropeptide Y neurons: similarities and differences. J Neurophysiol. 2011;106(3):1191–1202. doi: 10.1152/jn.00740.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]