Abstract
OBJECTIVE
Early menopause may be associated with higher cardiovascular disease (CVD) risk. Type 2 diabetes mellitus (T2DM), coupled with early menopause, may result in even greater CVD risk in women. We examined CVD risk in women with early compared with normal-age menopause, with and without T2DM overall, and by race/ethnicity.
RESEARCH DESIGN AND METHODS
We pooled data from the Atherosclerosis Risk in Communities study, the Multi-Ethnic Study of Atherosclerosis, and the Jackson Heart Study. We included women with data on menopausal status, menopausal age, and T2DM, excluding pre- or perimenopausal women and those with prevalent CVD. Outcomes included incident coronary heart disease (CHD), stroke, heart failure (HF), and atherosclerotic cardiovascular disease (ASCVD) (CHD or stroke). We estimated the risk associated with early (<45 years) compared with normal-age menopause using Cox proportional hazards models. Covariates included age, race/ethnicity, education, BMI, blood pressure, cholesterol, smoking, alcohol consumption, antihypertensive medication, lipid-lowering medication, hormone therapy use, and pregnancy history.
RESULTS
We included 9,374 postmenopausal women for a median follow-up of 15 years. We observed 1,068 CHD, 659 stroke, 1,412 HF, and 1,567 ASCVD events. T2DM significantly modified the effect of early menopause on CVD risk. Adjusted hazard ratios for early menopause and the outcomes were greater in women with T2DM versus those without (CHD 1.15 [95% CI 1.00, 1.33] vs. 1.09 [1.03, 1.15]; stroke 1.21 [1.04, 1.40] vs. 1.10 [1.04, 1.16]; ASCVD 1.29 [1.09, 1.51] vs. 1.10 [1.04, 1.17]; HF 1.18 [1.00, 1.39] vs. 1.09 [1.03, 1.16]). The modifying effect of T2DM on the association between early menopause and ASCVD was only statistically significant in Black compared with White women.
CONCLUSIONS
Early menopause was associated with an increased risk for CVD in postmenopausal women. T2DM may further augment the risk, particularly in Black women.
Introduction
One in three women dies as a result of cardiovascular disease (CVD) worldwide (1,2). The risk of CVD increases markedly after menopause (3). Menopause is characterized by a decrease in endogenous production of estrogen (4), which is associated with vascular dysfunction, increased blood pressure, redistribution of body fat toward abdominal areas, and hyperlipidemia, all of which increases CVD risk (4). The typical age range for menopausal transition is between 45 and 55 years; menopause onset at an age younger than 45 years is considered early menopause (5,6). Early menopause may be more detrimental to women’s cardiovascular health because of the early cessation of estrogen’s cardiovascular protection (3). Early menopause has been associated with increased risk of coronary heart disease (CHD) (7–9) and heart failure (HF) (10,11) and less consistently with stroke (9,12).
Midlife women with underlying metabolic disorders, such as type 2 diabetes mellitus (T2DM), face a higher risk of CVD. It is reported that postmenopausal women with T2DM are three times more likely to develop CHD or stroke than women without T2DM (13). The extent to which early menopause compounded by T2DM places women at an even higher risk of CVD is unknown. Additionally, despite previous evidence showing racial/ethnic heterogeneities in menopausal age (14), burden of T2DM (15), and postmenopausal CVD risk (15,16), it is unclear whether the effect of early menopause on CVD risk differs among races/ethnicities. Prior studies were limited by insufficient clinical events to determine whether early menopausal age contributes to a higher risk for CVD in racial minorities, such as non-Hispanic Blacks (Blacks) (8,10). Moreover, clinical studies examining the effect of menopausal transition and treatments on CVD risk often had limited or no representation of women with T2DM (17,18). Therefore, these studies were unable to shed light on the collective effect of early menopause and T2DM on subsequent CVD risk in postmenopausal women. In the current study, we used pooled data from three large multiethnic prospective studies to compare CVD risk in early menopausal women versus women who experienced menopause at normal age with or without T2DM, overall, and by racial group.
Research Design and Methods
Study Population
Participants were pooled from three major U.S. prospective studies of CVD: the Atherosclerosis Risk in Communities (ARIC) study, the Multi-Ethnic Study of Atherosclerosis (MESA), and the Jackson Heart Study (JHS). ARIC is a prospective epidemiologic study that enrolled 15,792 adults 45–64 years of age from four U.S. communities, including Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland (19,20). MESA is a population-based study that enrolled 6,815 adults 45–84 years of age from six U.S. communities, including Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota (21). JHS is a single-site, prospective, community-based study of African American adults recruited from Jackson, Mississippi (22). Detailed methods of each cohort’s study design, recruitment strategy, and visit protocols have been described previously (19–22) (Supplementary Appendix 1). The de-identified data were acquired from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center. This included most data elements from ARIC visit 1 through visit 5, MESA visit 1 through visit 5, and JHS visit 1 through visit 3. Written informed consent was provided by participants for the original three studies. In this analysis, we included women from ARIC visits 1, 3, and 4; MESA visit 1; and JHS visit 1 who were ≥45 years of age with information on menopausal status, age at menopause, T2DM (yes/no), and follow-up for CVD events. We did not include other visits from the three cohorts because of unavailable information on menopausal status, age at menopause, or key covariates of reproductive health information, such as ever used menopausal hormone therapy (MHT). This secondary analysis that involved existing de-identified data did not require review from the Tulane University institutional review board.
Measurements
T2DM was defined as a self-reported physician’s diagnosis, current insulin or hypoglycemic medication use, and/or a measured fasting glucose of ≥126 mg/dL.
Postmenopause, Early Menopause, and Menopausal Type
At the first, third, and fourth ARIC visits and JHS baseline, women were asked the following questions: “Have you had any menstrual periods during the past 2 years?” “Have you reached menopause?” “What was your age at your last menstrual period.” Women who had not had a menstrual period within the previous 2 years were classified as postmenopausal. (23,24). At MESA baseline, women were asked whether they had gone through menopause and were required to state the age at which they experienced menopause. Women who reported that they were going through menopause at baseline were asked to provide the date of their last menstrual period and the number of periods they had experienced in the past 12 months. Women who did not experience menstruation for ≥12 months before baseline were classified as being postmenopausal (11). Participants were classified as having early menopause if they experienced menopause before 45 years of age (10,11). Among postmenopausal women in all cohorts, those who reported menstrual cessation as a result of bilateral oophorectomy or radiation were classified as having surgical menopause, whereas those who reported cessation of menstrual bleeding that was not preceded by oophorectomy or radiation were classified as having natural menopause.
CVD Outcome Definitions and Ascertainments
For all three cohorts, incident CHD was defined as a myocardial infarction (MI), CHD death, or cardiac procedure (percutaneous coronary intervention, bypass surgery, or coronary revascularization). Stroke was defined as an ischemic or hemorrhagic stroke. Incident atherosclerotic cardiovascular disease (ASCVD) was defined as MI, CHD death, cardiac procedure(as just mentioned), or stroke. The adjudication process for events involved a panel to review hospitalization and death data per study protocols previously reported for all three cohorts (21,25,26). All events were adjudicated from medical records and death certificates for end point classification.
Covariates
We included baseline age, race/ethnicity (non-Hispanic White [White], Black, and other), education attainment, smoking status, alcohol drinking status, ever used MHT, and ever pregnant. This information was collected using standard questionnaires from the three studies. We also included baseline health indicators, including blood pressure, total cholesterol, and BMI. Detailed measurement procedures for the ARIC, MESA, and JHS can be found in previous publications (21,25,26).
Analysis
From an original 25,725 participants (ARIC, 15,028; MESA, 6,814; JHS, 3,883), we excluded men, women with pre- or perimenopause or with missing menopausal status, and ARIC and JHS overlap participants. Among postmenopausal women, we further excluded women with prevalent CVD; those with missing key covariates, T2DM status, or menopausal age; or those with missing CVD outcomes. We had a total number of 9,374 postmenopausal women (T2DM, 1,237; non-T2DM, 8,137) eligible for analysis. A detailed sample selection process from the cohorts, individually and overall, is shown in Supplementary Fig. 1. Missingness for covariates was minimal (<0.07%), except for alcohol drinking (9% missing), menopausal type (4% missing), and ever used MHT (11% missing). We conducted multiple imputations for these three covariates.
We used χ2 and t tests to compare the differences of characteristics between postmenopausal women with and without T2DM. CVD event rates were calculated per 1,000 person-years. Poisson regression was conducted to compare the difference in incidence rate between menopause at <45 and ≥45 years of age. The association between early menopause and CVD outcomes was examined in Cox proportional hazards models. The model was first adjusted for age and race/ethnicity and then further adjusted for CVD risk factors, including smoking status, alcohol drinking status, blood pressure, total cholesterol, BMI, ever used MHT, ever pregnant, and cohort indicator. Interaction terms between early menopause and T2DM were added to models to test the potential modifying effect of T2DM on early menopause and CVD outcomes. The primary analysis included the overall sample; secondary analyses were conducted in race-specific subgroups. We also conducted a series of sensitivity analyses, including assessment of the relationship between early menopause and CVD risk in individuals <70 years old, according to fasting glucose level and in each individual cohort. All analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC).
Results
Table 1 presents the descriptive characteristics of the pooled cohort. Mean age was 58 years; the T2DM group was older than the non-T2DM group (59 vs. 58 years). Self-reported race among the postmenopausal women in this study was 58%, 32%, and 10% for White, Black, and other, respectively. In the T2DM group, there was a higher proportion of Black than White women or women of other racial groups (52% vs. 35% and 13%, respectively). In the non-T2DM group, there were more White than Black women or women of other racial groups (61% vs. 29% and 10%, respectively). Compared with women without T2DM, individuals with T2DM were more likely to have less than a high school education (65% vs. 52%), be obese (61% vs. 31%), be hypertensive (55% vs. 35%), have experienced early menopause (41% vs. 36%), have had surgical menopause (42% vs. 37%), and have a pregnancy history (94% vs. 92%). However, they were less likely to be current smokers (14% vs. 19%) or current alcohol users (33% vs. 57%).
Table 1.
Baseline characteristics of postmenopausal women by T2DM from three cohorts
| All (n = 9,374) | T2DM (n = 1,237) | Non-T2DM (n = 8,137) | P | |
|---|---|---|---|---|
| Age (years) | 57.7 ± 8.6 | 58.9 ± 8.4 | 57.6 ± 8.6 | <0.0001 |
| Race/ethnicity | <0.0001 | |||
| Non-Hispanic White | 5,391 (57.5) | 436 (35.2) | 4,955 (60.9) | |
| Non-Hispanic Black | 3,016 (32.2) | 641 (51.8) | 2,375 (29.2) | |
| Other | 967 (10.3) | 160 (13.0) | 807 (9.9) | |
| Education | <0.0001 | |||
| High school or less | 5,027 (53.7) | 804 (65.1) | 4,223 (52.0) | |
| More than high school | 4,332 (46.3) | 432 (35.0) | 3,900 (48.0) | |
| Current smoking | 1,709 (18.2) | 176 (14.2) | 1,533 (18.9) | <0.0001 |
| Current alcohol drinking | 5,064 (54.0) | 407 (32.9) | 4,657 (57.2) | <0.0001 |
| BMI | ||||
| BMI (kg/m2) | 28.5 ± 6.3 | 32.4 ± 6.7 | 27.9 ± 6.0 | <0.0001 |
| BMI ≥30 kg/m2 | 3,263 (34.8) | 747 (60.5) | 2,516 (30.9) | <0.0001 |
| Blood pressure | ||||
| SBP (mmHg) | 125.1 ± 21.2 | 133.3 ± 22.5 | 123.9 ± 20.7 | <0.0001 |
| DBP (mmHg) | 71.1 ± 10.6 | 71.6 ± 10.9 | 71.0 ± 10.5 | 0.05 |
| SBP ≥140 mmHg or DBP ≥90 mmHg | 3,535 (37.8) | 680 (55.1) | 2,855 (35.1) | <0.0001 |
| Cholesterol | ||||
| Total (mg/dL) | 212.2 ± 40.9 | 212.3 ± 47.0 | 212.2 ± 39.9 | 0.96 |
| Total cholesterol ≥200 mg/dL | 5,542 (59.6) | 689 (57.5) | 4,853 (59.9) | 0.11 |
| Antihypertensive medication | 3,167 (33.8) | 743 (60.2) | 2,424 (29.8) | <0.0001 |
| Lipid-lowering medication | 2,294 (24.6) | 494 (40.2) | 1,800 (22.2) | <0.0001 |
| Menopausal age (years) | 45.4 ± 7.0 | 44.7 ± 7.6 | 45.6 ± 6.9 | <0.0001 |
| Early menopause (<45 years) | 3,447 (36.8) | 511 (41.4) | 2,936 (36.1) | 0.0004 |
| Menopausal type | ||||
| Natural | 5,757 (61.4) | 703 (56.8) | 5,054 (62.1) | 0.001 |
| Surgical | 3,519 (37.5) | 516 (41.7) | 3,003 (36.9) | |
| Not specified | 98 (1.1) | 18 (1.5) | 80 (1.0) | |
| Ever used hormone therapy | 5,051 (54.1) | 591 (48.1) | 4,460 (55.1) | <0.0001 |
| Ever pregnant | 8,609 (91.9) | 1,158 (93.7) | 7,451 (91.6) | 0.01 |
Data are mean ± SD or n (%). Missing n for covariates: education, 15; smoking, 4; BMI, 5; blood pressure, 10; cholesterol, 69; antihypertensive medication, 11; lipid-lowering medication, 40; and ever pregnant, 5. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Baseline characteristics by racial group are displayed in Supplementary Table 1. Overall, postmenopausal women from other racial minorities were older and more likely to have less than a high school education than White and Black women at baseline. Black women had higher rates of obesity and hypertension (i.e., systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg) compared with women from White or other racial groups. White women, however, were more likely to have elevated total cholesterol, be current smokers, and be current alcohol drinkers than the other two racial groups. Black women reported menopause onset at a younger age and had the highest proportion of surgical menopause among the three racial groups in the cohort. White women were more likely to use MHT than the Black and other racial groups. Across all racial groups, women with T2DM were older and more likely to have less than a high school education. They were more likely to be obese and hypertensive, less likely to be smokers or alcohol drinkers, and less likely to use MHT at baseline than women without T2DM (Supplementary Table 1).
In Supplementary Table 2, we present baseline characteristics in early versus normal-age menopause. Compared with women with normal-age menopause, women with early menopause were younger and more likely to have less than a high school education, be a smoker, have T2DM, be obese, and have undergone surgical menopause. However, women with early menopause were less likely to be a current alcohol drinker or hypertensive.
Baseline characteristics by cohort are presented in Supplementary Table 3. There were fewer participants from JHS (only Black women) compared with the other two cohorts. Compared with ARIC or MESA, JHS participants had a higher proportion of obesity, T2DM, antihypertensive medication usage, early menopause, and surgical menopause.
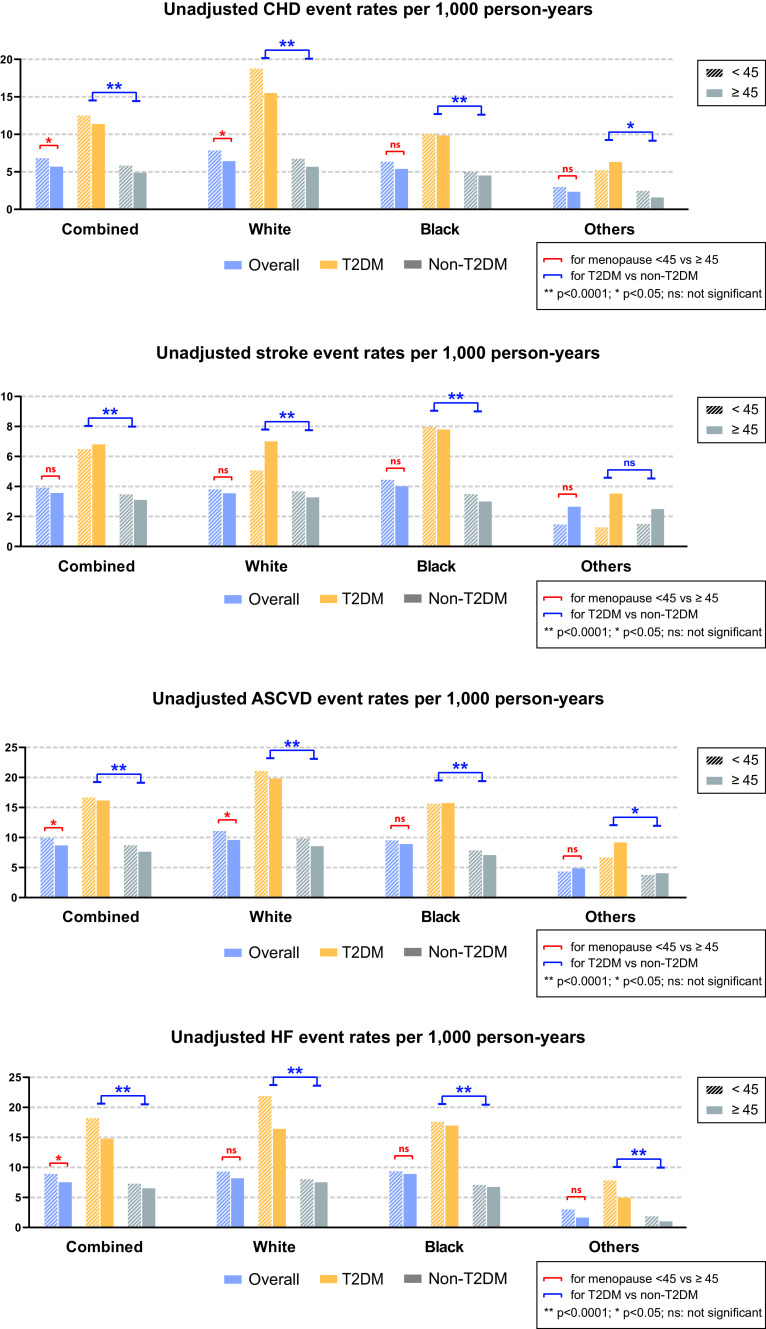
During a median follow-up of 15 years for 9,374 postmenopausal women, 1,068 CHD, 659 stroke, 1,412 HF, and 1,567 ASCVD events were observed. Women with early menopause experienced significantly higher crude CHD, ASCVD, and HF event rates (incidence per 1,000 person-years) than those who had menopause at normal age (Fig. 1). Women with T2DM who reported early menopause showed significantly higher incident rates across all outcomes than those who reported menopause at normal age with or without T2DM (Fig. 1). This remained the case after stratifying by race.
Figure 1.
Unadjusted event rates (per 1,000 person-years) of CHD, stroke, ASCVD, and HF.
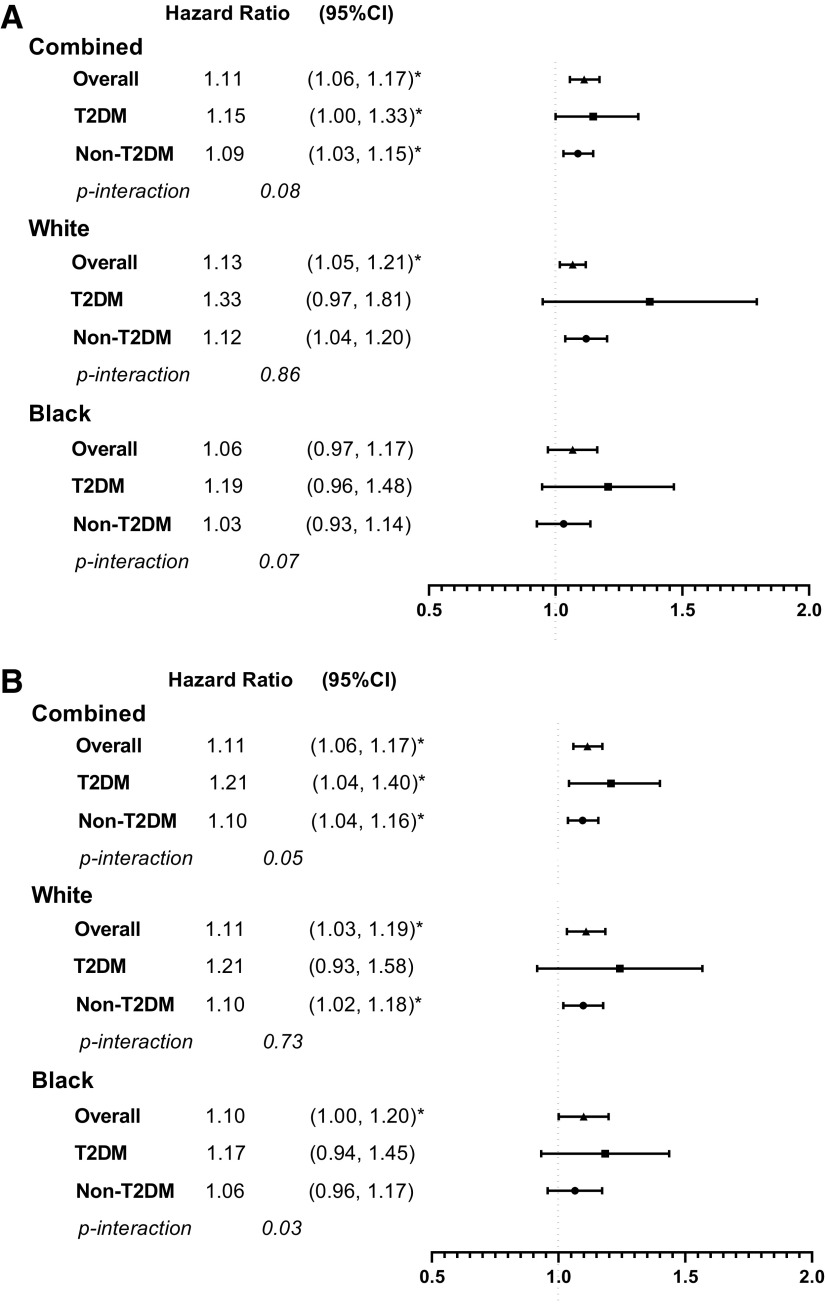
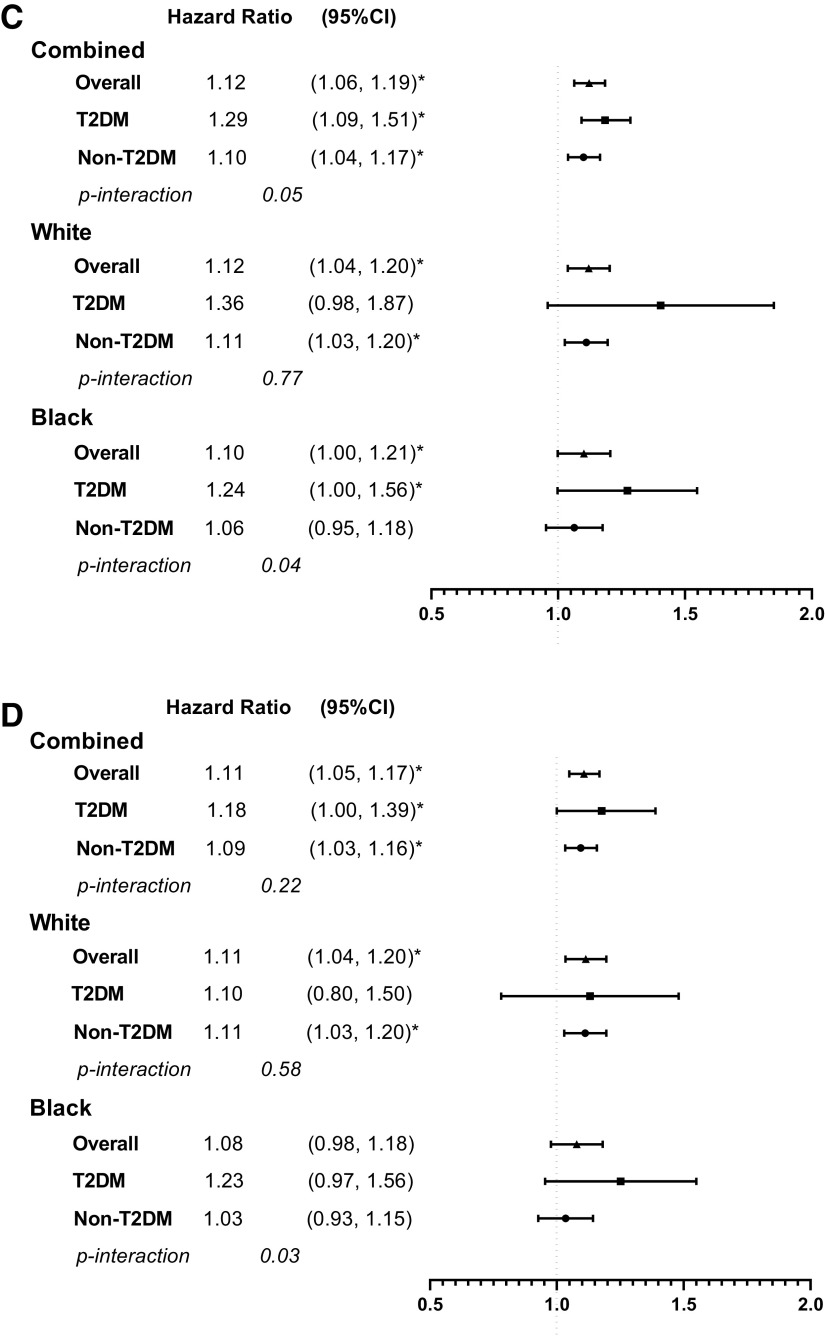
Age- and race-adjusted associations between early menopause and all outcomes were statistically significant (each P < 0.05) (Supplementary Table 4). In fully adjusted models, the association between early menopause and risk for CHD (hazard ratio [HR] 1.11 [95% CI 1.06, 1.17]), stroke (1.11 [1.06, 1.17]), ASCVD (1.12 [1.06, 1.19]), and HF (1.09 [1.03, 1.16]) remained significant (Fig. 2). Notably, T2DM significantly modified the relationship between early menopause and CHD, stroke, and ASCVD (P for interaction ≤0.05). In fully adjusted Cox models, HRs for early menopause and the outcomes were greater in women with than those without T2DM (CHD, 1.15 [1.00, 1.33] vs. 1.09 [1.03, 1.15]; stroke, 1.21 [1.04, 1.40] vs. 1.10 [1.04, 1.16]; ASCVD, 1.29 [1.09, 1.51] vs. 1.10 [1.04, 1.17]; HF, 1.18 [1.00, 1.39] vs. 1.09 [1.03, 1.16]) (Fig. 2).
Figure 2.
Adjusted HRs of early menopause and CHD (A), stroke (B), ASCVD (C), and HF (D). HRs were adjusted for age, race, cohort indicator, education, smoking status, alcohol drinking status, BMI, mean blood pressure, mean total cholesterol, antihypertensive medication, lipid-lowering medication, ever used hormone therapy, pregnancy history, and T2DM. *P < 0.05.
In fully adjusted analyses stratified by race, early menopause was significantly associated with increased risk of CHD, stroke, ASCVD, and HF in White women. In Black women, early menopause was significantly associated with an increased risk of stroke and ASCVD (Fig. 2). In women of other racial groups, there was a trend of increased risk of CHD, stroke, ASCVD, and HF with early menopause, despite absent statistical significance (Supplementary Table 5). The modifying effect of T2DM on the association between early menopause and CVD was especially evident in Black women (P value for interaction between early menopause and T2DM was 0.07, 0.03, 0.04, and 0.03 for CHD, stroke, ASCVD, and HF, respectively). The risk of ASCVD associated with early menopause was higher in Black women with T2DM than Black women without T2DM (Fig. 2).
To rule out the heightened CVD risk as a result of chronological aging in the elderly rather than our exposures of interest (early menopause and T2DM), we conducted a sensitivity analysis for a cohort of women <70 years old. The association between early menopause and risk of CVD was attenuated. However, the modifying effect of T2DM on the association remained significant and was of greater magnitude (Supplementary Table 6).
We also examined the association between early menopause and CVD risk by fasting glucose level (<100, 100–125, or ≥126 mg/dL). We found that the magnitude of the association increased as the glucose level increased (i.e., glycemic control) (Supplementary Table 7). When replicating the analyses in each original cohort, we observed similar findings to the pooled cohort (Supplementary Table 8). Similar to results from the pooled analysis, early menopause was associated with significantly increased risk of CHD, stroke, ASCVD, and HF, specifically in ARIC and MESA. In each cohort, those with T2DM showed higher HRs of early menopause and CVD outcomes than those without T2DM (Supplementary Table 8). The modifying effect of T2DM was especially pronounced in JHS, which consisted of only Black women, among whom T2DM was more prevalent than other racial groups in the study (Supplementary Tables 1 and 8).
Conclusions
The main finding of this study is the augmentation of the association between early menopause and the incidence of CVD by T2DM. The hazard of CVD associated with early menopause was more evident in women with T2DM than those without, particularly among Black participants. In our study, more Black women had poor glycemic control (i.e., fasting glucose ≥136 mg/dL) than White women (5.5% vs. 1.7%). The nonsignificant modifying effect of T2DM in White women was possibly due to the smaller number of individuals with T2DM and/or a relatively better glycemic control. Additionally, the association between early menopause and CVD risk is similarly strong across Black and White women. T2DM is the most common chronic disease in postmenopausal women and a significant predictor of CVD (27); however, few studies have examined CVD risk in postmenopausal women with preexisting T2DM, especially among those with early menopause. T2DM accelerates reproductive aging and contributes to premature ovarian failure by various mechanisms, including lower levels of sex hormone–binding globulin, estradiol, hypothalamic-pituitary hypofunction, and hyperandrogenism (27). Menopause, on the other hand, is associated with increased abdominal fat, insulin resistance, and excess hepatic lipid accumulation (27), which increase risks of CHD and stroke in postmenopausal years (28). Moreover, menopausal estrogen deficiency alters glucose homeostasis. Glycemic control could be further compromised by increased cortisol during menopause, which favors atherosclerosis and is associated with cardiovascular consequences in postmenopausal years (29). T2DM and early menopause have been individual predictors of morbidity and mortality as a result of CVD in women. The current study underscores the importance of both early menopause and T2DM with regard to CVD risk among women and identifies for clinicians a new high-risk subgroup of women to monitor and treat to reduce the burden of postmenopausal CVD.
Our study extends prior findings by confirming a positive association between early menopause and stroke and ASCVD. Estrogens increase vasodilatation (26), inhibit the response of blood vessels to injury, and delay the development of atherosclerosis (25). Early loss of estrogen impairs vascular function and increases inflammatory cytokine production at younger ages, which further damages vascular function (27) and leads to ASCVD. However, observational findings about the effect of early menopause on stroke have been inconclusive. Two meta-analyses reported a statistically nonsignificant hazard of stroke following early menopause (9,30), which was also reported in three separate studies (31–33). Two of these studies, however, were restricted to women who never used MHT (31,33), while another was limited by a small number of stroke events (32). Conversely, the Framingham Heart Study, the Nurses’ Health Study, and a Chinese cohort study all reported that women with early menopause had a significantly elevated risk for ischemic or hemorrhagic stroke (34–36). In two meta-analyses, researchers also found that early menopause is significantly associated with increased risk of stroke (12,37). Here, we leveraged three large longitudinal cohorts of CVD and confirmed a significant positive relationship between early menopause and stroke or ASCVD. Furthermore, our findings on the positive association between early menopause and HF aligned with previous reports (10,11).
We also addressed a limitation of the literature where most studies of postmenopausal CVD risk were predominantly based on a single-race or a combined racial/ethnic sample (31–36). Timing and reasons for reaching menopause differ by race/ethnicity. Black women reach menopause earlier than White women (14) and are more likely to receive premenopausal hysterectomy with oophorectomy, leading to early surgical menopause (38). There is also a large heterogeneity in the risk and burden of CVD by ethnicity among women, with Black women facing significantly higher rates of T2DM and subsequent CVD than White women (39). In our study, we provided a racial-specific perspective in the relationship between early menopause and CVD risk.
The strengths of this study include the use of three large and well-characterized multiethnic cohorts, which provides an opportunity to investigate the relationship between early menopause and CVD by T2DM status and race. The three cohorts also included a standardized evaluation of risk factors and ascertainment of CVD events that were adjudicated by end point committees. Additionally, the participant-level data from the established CVD cohorts enabled us to harmonize variables using common definitions, coding, and cutoff points, which is superior to meta-analysis of published aggregated results. Several limitations of the study warrant consideration. First, the inadequate power from participants who did not identify as non-Hispanic White or non-Hispanic Black (i.e., other race/ethnicity) precludes a precise estimation of the effect of early menopause as well as the potential modifying effect of T2DM on CVD risk in this subpopulation. This highlights the importance of including more racial minority women in future epidemiological studies to support robust analysis regarding women’s postmenopausal cardiovascular health. Second, the self-reported menopausal age may be subject to error and may have resulted in misclassification. The precision of recollecting age at menopause is likely to decrease as time since menopause increases (40). This may dilute the relative hazard estimates, as women who had undergone early menopause may subsequently report an older age at menopause (10). Third, it remained unanswered in the literature whether early cessation of reproductive function etiologically increases risk of CVD or whether latent CVD causes reproductive aging (or both) (3). On the basis of our data, we were unable to assess directionality of the association, but through exclusion of those with prevalent CVD and adjustment for conventional CVD risks in analysis, we were able to examine the role of early menopause in CVD risk.
In conclusion, early menopause is associated with increased risk of CHD, stroke, ASCVD, and HF in postmenopausal women. T2DM further augments the CVD risk in early menopausal women, specifically the Black subgroup. Early menopause is an important determinant of future CVD risk and should be included in CVD risk stratification and assignment of early interventions for postmenopausal women, especially among those with T2DM.
Article Information
Acknowledgments. The authors thank the investigators, staff, and participants of ARIC, MESA, and JHS for their contributions. They also thank the staff of the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center for coordinating the data access.
Funding. Y.Y. and M.K.-W. were supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant K12-HD-043451 of the Building Interdisciplinary Research Careers in Women’s Health. F.M.-J. was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK-107444 and DK-074970, a U.S. Department of Veterans Affairs Merit Award (BX003725), and the Tulane Center of Excellence in Sex-Based Biology and Medicine. A.H.A. was supported by National Institute of Diabetes and Digestive and Kidney Disease grants R01-DK-104730 and R01-DK-107566, National Institutes of Health grant P20-GM-109036, and the Tulane University Translational Science Institute.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.Y. designed the study and contributed to the data management and analysis, results interpretation, and drafting, reviewing, and editing of the manuscript. Z.C. contributed to the data management, analysis, and figure making. R.L.B. provided statistical advice and contributed to manuscript reviewing and editing. M.K.-W., A.H.A, V.A.F, and F.M.-J. provided critical comments on the study and reviewed and edited the manuscript. Y.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at Tulane Health Sciences Days 2021, New Orleans, LA, 14–15 April 2021.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.15135006.
References
- 1. Garcia M, Mulvagh SL, Merz CN, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res 2016;118:1273–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manson JE, Woodruff TK. Reproductive health as a marker of subsequent cardiovascular disease: the role of estrogen. JAMA Cardiol 2016;1:776–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. North American Menopause Society . The 2012 hormone therapy position statement of: the North American Menopause Society. Menopause 2012;19:257–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santoro N. Mechanisms of premature ovarian failure. Ann Endocrinol (Paris) 2003;64:87–92 [PubMed] [Google Scholar]
- 6. Shifren JL; NAMS Recommendations for Clinical Care of Midlife Women Working Group . The North American Menopause Society recommendations for clinical care of midlife women. Menopause 2014;21:1038–1062 [DOI] [PubMed] [Google Scholar]
- 7. Savonitto S, Morici N, Franco N, et al.; LADIES ACS Investigators . Age at menopause, extent of coronary artery disease and outcome among postmenopausal women with acute coronary syndromes. Int J Cardiol 2018;259:8–13 [DOI] [PubMed] [Google Scholar]
- 8. Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause 2012;19:1081–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol 2016;1:767–776 [DOI] [PubMed] [Google Scholar]
- 10. Appiah D, Schreiner PJ, Demerath EW, Loehr LR, Chang PP, Folsom AR. Association of age at menopause with incident heart failure: a prospective cohort study and meta-analysis. J Am Heart Assoc 2016;5:e003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ebong IA, Watson KE, Goff DC Jr., et al. Age at menopause and incident heart failure: the Multi-Ethnic Study of Atherosclerosis. Menopause 2014;21:585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Honigberg MC, Zekavat SM, Aragam K, et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA 2019;322:2411–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldberg RJ, Larson M, Levy D. Factors associated with survival to 75 years of age in middle-aged men and women. The Framingham Study. Arch Intern Med 1996;156:505–509 [PubMed] [Google Scholar]
- 14. McKnight KK, Wellons MF, Sites CK, et al. Racial and regional differences in age at menopause in the United States: findings from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Obstet Gynecol 2011;205:353.e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors–an Endocrine Society scientific statement. J Clin Endocrinol Metab 2012;97:E1579–E1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barinas-Mitchell E, Duan C, Brooks M, et al. Cardiovascular disease risk factor burden during the menopause transition and late midlife subclinical vascular disease: does race/ethnicity matter? J Am Heart Assoc 2020;9:e013876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller VM, Naftolin F, Asthana S, et al. The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause 2019;26: 1071–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruppert K, Cauley J, Lian Y, Zgibor JC, Derby C, Solomon DH. The effect of insulin on bone mineral density among women with type 2 diabetes: a SWAN pharmacoepidemiology study. Osteoporos Int 2018;29:347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chambless LE, Folsom AR, Sharrett AR, et al. Coronary heart disease risk prediction in the Atherosclerosis Risk in Communities (ARIC) study. J Clin Epidemiol 2003;56:880–890 [DOI] [PubMed] [Google Scholar]
- 20. Prabhakar C, Chicken E, McGee D. Time scales in epidemiological analysis: an empirical comparison. Int J Stat Probab 2016;5:91–101 [Google Scholar]
- 21. Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881 [DOI] [PubMed] [Google Scholar]
- 22. Taylor HA Jr., Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis 2005;15:S6-4-17. [PubMed] [Google Scholar]
- 23. Gurka MJ, Vishnu A, Santen RJ, DeBoer MD. Progression of metabolic syndrome severity during the menopausal transition. J Am Heart Assoc 2016;5:e003609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nabulsi AA, Folsom AR, White A, et al.; The Atherosclerosis Risk in Communities Study Investigators . Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. N Engl J Med 1993;328:1069–1075 [DOI] [PubMed] [Google Scholar]
- 25. The ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 26. Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 2004;328:131–144 [DOI] [PubMed] [Google Scholar]
- 27. Sekhar TV, Medarametla S, Rahman A, Adapa SS. Early menopause in type 2 diabetes - a study from a South Indian tertiary care centre. J Clin Diagn Res 2015;9:OC08–OC10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samargandy S, Matthews KA, Brooks MM, et al. Abdominal visceral adipose tissue over the menopause transition and carotid atherosclerosis: the SWAN Heart Study. Menopause 2021;28: 626–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cagnacci A, Cannoletta M, Caretto S, Zanin R, Xholli A, Volpe A. Increased cortisol level: a possible link between climacteric symptoms and cardiovascular risk factors. Menopause 2011;18: 273–278 [DOI] [PubMed] [Google Scholar]
- 30. Roeters van Lennep JE, Heida KY, Bots ML; Collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders . Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol 2016;23:178–186 [DOI] [PubMed] [Google Scholar]
- 31. Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause 2010;17:506–510 [DOI] [PubMed] [Google Scholar]
- 32. Choi SH, Lee SM, Kim Y, Choi NK, Cho YJ, Park BJ. Natural menopause and risk of stroke in elderly women. J Korean Med Sci 2005;20:1053–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med 1999;159:1061–1066 [DOI] [PubMed] [Google Scholar]
- 34. Shen L, Song L, Liu B, et al. Effects of early age at natural menopause on coronary heart disease and stroke in Chinese women. Int J Cardiol 2017;241:6–11 [DOI] [PubMed] [Google Scholar]
- 35. Ley SH, Li Y, Tobias DK, et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc 2017;6:e006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham Heart Study. Stroke 2009;40:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu D, Chung HF, Dobson AJ, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health 2019;4:e553–e564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health 2009;99:300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Regensteiner JG, Golden S, Huebschmann AG, et al.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Functional Genomics and Translational Biology, and Council on Hypertension . Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American Heart Association. Circulation 2015;132:2424–2447 [DOI] [PubMed] [Google Scholar]
- 40. Hahn RA, Eaker E, Rolka H. Reliability of reported age at menopause. Am J Epidemiol 1997;146:771–775 [DOI] [PubMed] [Google Scholar]