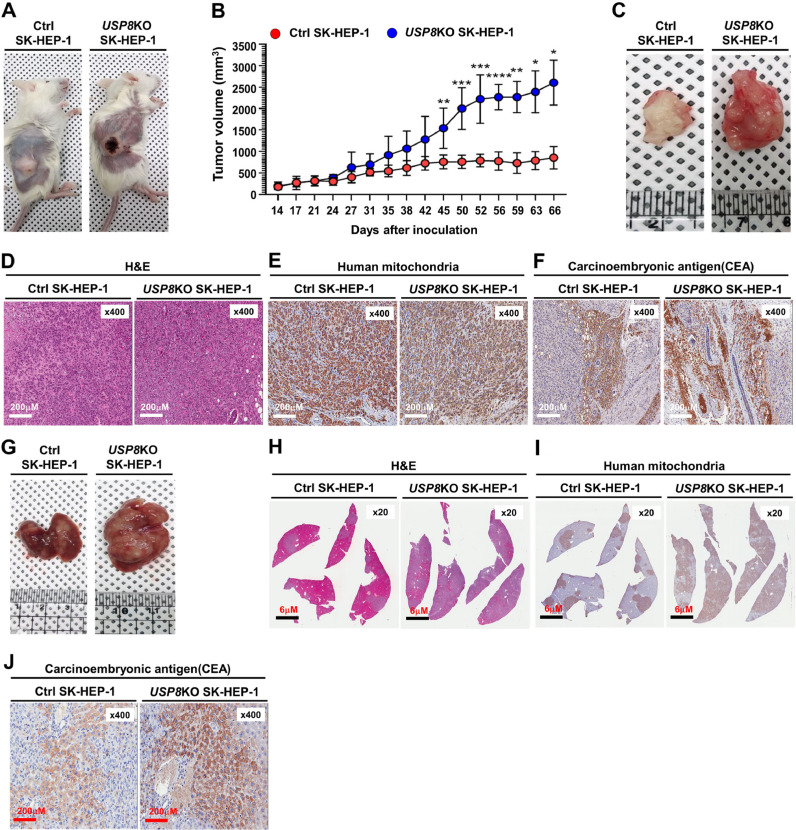
Fig. 7.
Tumorigenicity and metastasis of USP8KO SK-HEP-1 cells are increased in NSG mice. (A) The primary tumors (human xenografts) at day 66 post subcutaneous injection of Ctrl SK-HEP-1 or USP8KO SK-HEP-1 cells in NSG mice. (B) Xenograft tumor volumes of each group (Ctrl SK-HEP-1 injected group; USP8KO SK-HEP-1 cells injected group) were measured, at indicated times after post-injected day. Values are mean ± SEM. (n = 10 each, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 significant compared with Ctrl SK-HEP-1 and USP8KO SK-HEP-1, Student's t-test). (C) Macroscopic primary tumors formed after the subcutaneous injection of Ctrl SK-HEP-1 or USP8KO SK-HEP-1 cells at day 66 post injection in NSG mice. (D-F) The histological and pathological features of primary subcutaneous tumor cells derived from NSG mice xenografted with Ctrl SK-HEP-1 or USP8KO SK-HEP-1 cells were assessed by H&E staining (D), human mitochondria staining (E), and carcinoembryonic antigen (CEA) (F). (G) Macroscopic metastatic liver tumors formed by the subcutaneous injection of Ctrl SK-HEP-1 or USP8KO SK-HEP-1 cells day 66 post-injection in NSG mice. (H-J) The histological and pathological features of metastatic liver tumor cells derived from NSG mice xenografted with Ctrl SK-HEP-1 or USP8KO SK-HEP-1 cells were assessed by H&E staining (H), human mitochondria staining (I), and carcinoembryonic antigen (CEA) (J).