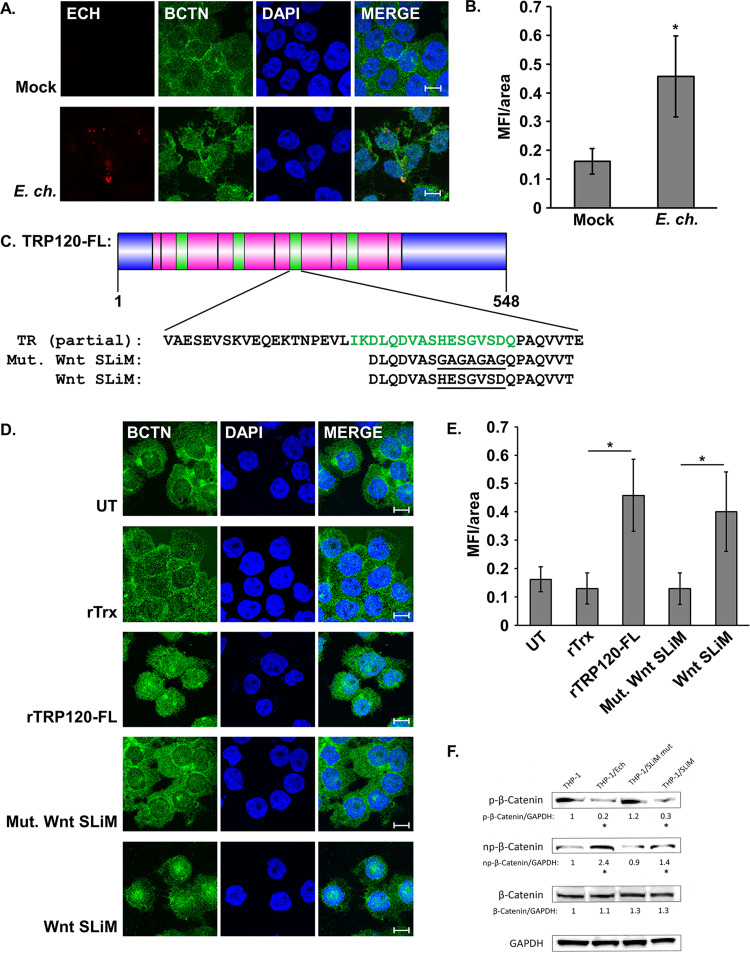
FIG 5.
TRP120 activated canonical Wnt signaling through a Wnt SLiM. (A) E. chaffeensis infection stimulates β-catenin nuclear translocation. THP-1 cells were infected or mock infected with E. chaffeensis (MOI 100), harvested 3 hpi, immunostained for E. chaffeensis (red) and β-catenin (BCTN, green), and visualized by confocal fluorescence microscopy. Scale bar = 10 μm. (B) Wnt pathway activation was quantified by measuring mean nuclear fluorescence intensity per area (MFI/area) of β-catenin in infected versus mock-infected cells. (C) Protein domain map of TRP120-FL. The Wnt SLiM is indicated in green. Sequences of peptides used to investigate Wnt pathway activation are aligned with respective region in the TRD (pink). The N terminus and C terminus are indicated in blue. (D) rTRP120-FL and a Wnt SLiM peptide stimulate β-catenin activation. THP-1 cells were treated with indicated recombinant proteins or synthetic peptides (2 μg/ml) for 3 h, immunostained for β-catenin (green), and visualized by confocal fluorescence microscopy. Scale bar = 10 μm. (E) Quantification of Wnt pathway activation by different treatment groups was measured as described above. Values are an average from three independent experiments plus or minus standard deviation. *, P < 0.05. (F) Western immunoblot demonstrating decreased phosphorylation of β-catenin in response to E. chaffeensis or Wnt SLiM peptide at 3 h postincubation compared to uninfected THP-1 cells and cells incubated with mutant Wnt SLiM peptide. Densitometry values are shown below each respective band and are shown as fold change normalized to GAPDH. *, P < 0.05. Significance compared to uninfected control was calculated from three individual experiments (n = 3).