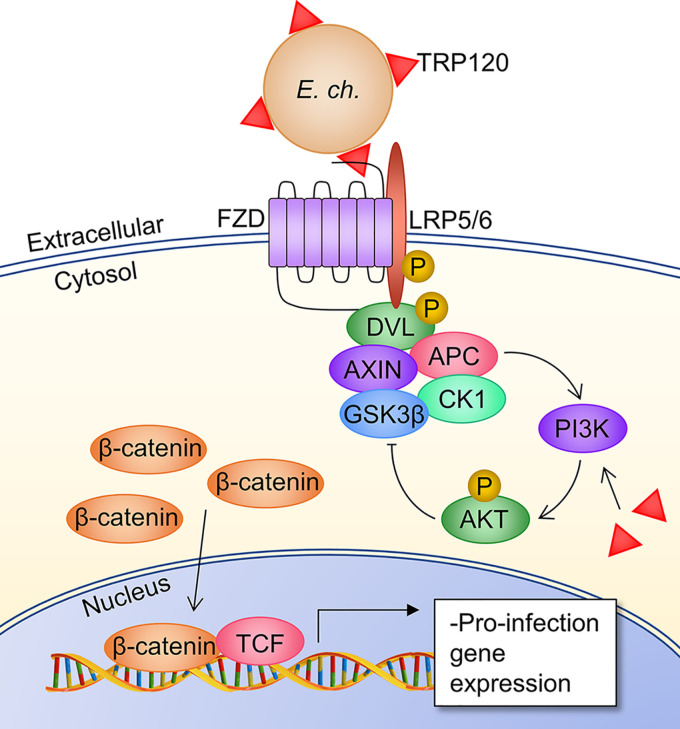
FIG 7.
Proposed model of E. chaffeensis manipulation of canonical Wnt signaling by TRP120. DC ehrlichia surface-expressed TRP120 directly engages one or more Fzd receptors at the extracellular conserved CRD through a Wnt SLiM repeated in the TRP120 TRD. This results in recruitment of coreceptor LRP5 and activation of canonical Wnt signaling through disassembly of the β-catenin destruction complex (consisting of Axin, APC, GSK3β, and CK1), which allows accumulation of β-catenin in the cytoplasm and its subsequent nuclear translocation for activation of Wnt target genes. These genes are characterized as pro-E. chaffeensis infection, as inhibition of signaling impairs bacterial infection (22). Furthermore, TRP120 that is secreted into the host cytoplasm from the E. chaffeensis morula during infection drives inhibition of the β-catenin destruction complex through phosphatidylinositol 3-kinase (PI3K)/AKT signaling which amplifies canonical Wnt signaling and promotes infection through suppression of autophagy (42).