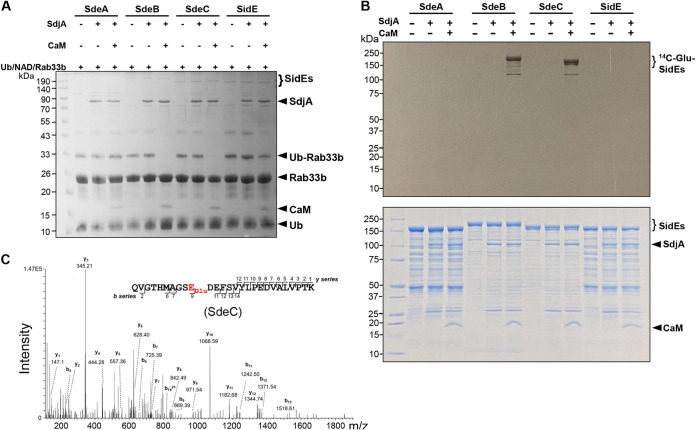
FIG 4.
SdjA attacks the mART motif of SdeB and SdeC by glutamylation. (A) Inhibition of SdeB and SdeC by SdjA requires CaM. Recombinant SdjA was incubated with glutamate and each protein of the SidE family with or without CaM for 2 h at 37°C. The activity of these ubiquitin ligases was assessed by adding a reaction mixture containing ubiquitin, NAD, and Rab33b. Note the loss of activity by SdeB and SdeC in the presence of CaM (middle two sets of samples). (B) Transfer of glutamate to SdeB and SdeC by SdjA. Recombinant SdjA was incubated with each protein of the SidE family in reaction mixtures containing [14C]glutamate with or without CaM at 37°C for 2 h. After SDS-PAGE, gels stained with Coomassie brilliant blue (bottom) were dried, and the incorporation of radiolabeled glutamate into these ubiquitin ligases was detected by autoradiograph (top). Note the CaM-dependent modification of SdeB and SdeC. (C) SdjA induced glutamylation on Glu843 within the mART motif of SdeC. His6-SdeC was incubated with SdjA, glutamate, and CaM. The protein band corresponding to His6-SdeC was excised from SDS-PAGE gels and analyzed by mass spectrometry, which detected a glutamylation in the fragment Q834VGTHMAGSEDEFSVYLPEDVALVPTK860. The tandem mass (MS/MS) spectrum shows the fragmentation profile of the modified peptide Q834VGTHMAGSEGluDEFSVYLPEDVALVPTK860, including ions b9 and b11, which confirms the modification site at the Glu843 residue.