FIG 5.
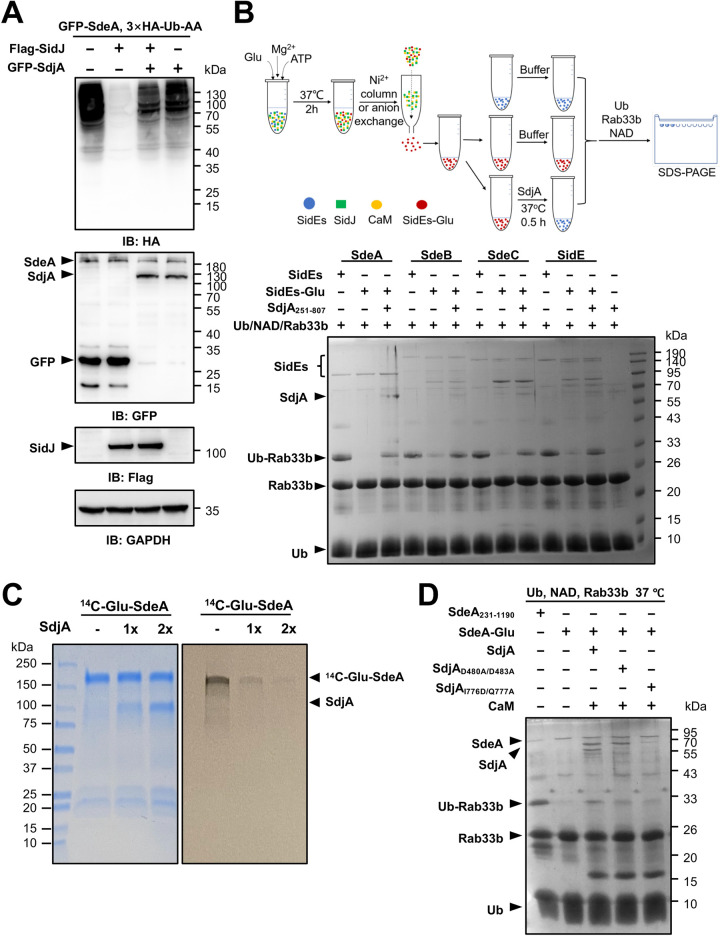
SdjA functions as a deglutamylase to reverse the modification induced by SidJ on SdeA. (A) SdjA antagonizes the inhibitory effects of SidJ on the ubiquitination activity of SdeA. HEK293 cells were transfected with the indicated plasmid combinations, and ubiquitination by HA-Ub-AA was probed by immunoblotting with the HA-specific antibody (top). The expression of relevant proteins was probed with the appropriate antibodies (middle and bottom). Note that coexpression of SdjA with SidJ restored ubiquitination induced by SdeA (compared the second and third lanes). (B) A central fragment of SdjA (SdjA251–807) exhibited deglutamylase activity. Proteins of the SidE family were individually glutamylated by SidJ following a procedure depicted in the top panel. In each case, glutamylated protein was produced by reactions that contain SidJ, ATP, and glutamate. Modified proteins were purified; one half of each modified protein was incubated with SdjA251–807 prior to being assayed for the ubiquitin ligase activity with a cocktail that contained NAD, ubiquitin, and Rab33b, and the second half was used directly in the activity assay. For each member, a reaction with native active protein was included as a control. Note that SdjA251–807 restored the activity of all members of the SidE family that had been inactivated by SidJ as indicated by the formation of Ub-Rab33b (compare the second and third lanes for each protein) (bottom). (C) Deglutamylation of [14C]Glu-SdeA by GST-SdjA37–782. [14C]Glu-SdeA isolated from a reaction mixture containing SidJ, CaM, SdeA, and [14C]Glu was incubated with two different amounts of GST-tagged SdjA for 2 h at 37°C. Samples resolved by SDS-PAGE were subjected to autoradiography (top) and the proteins were detected by Coomassie brilliant blue staining (bottom). (D) A SdjA mutant defective in glutamylase activity retains deglutamylase activity. Glutamylated SdeA obtained by a procedure described in panel B was incubated with SdjA or its mutant defective in glutamylase activity or in the IQ motif prior to being assayed for the ubiquitin ligase activity with reagents listed at the top. Note that SdjAD480A/D483A, defective in the pseudokinase domain essential for the glutamylase activity, retains the ability to restore the activity of Glu-SdeA (fourth lane).