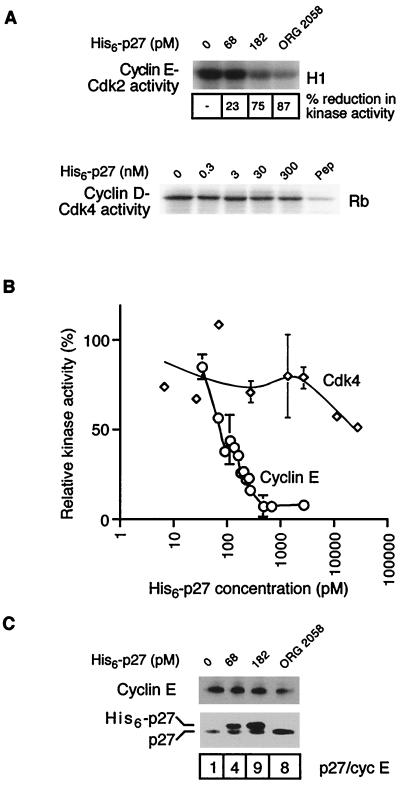
FIG. 3.
Effect of His6-p27 addition on cyclin-CDK complex activity and composition. Recombinant His6-p27 was expressed in E. coli, purified on Ni-NTA beads, and incubated with extract from proliferating T-47D cells in vitro over a range of concentrations of His6-p27. (A) Cyclin E and Cdk4 were immunoprecipitated following incubation with His6-p27, the immune complexes were resuspended in kinase buffer, and associated kinase activity was determined by the transfer of [32P]phosphate to histone H1 (cyclin E) or GST-Rb (Cdk4) as described previously (40). To indicate the level of nonspecific Rb kinase activity in Cdk4 assays, a sample was immunoprecipitated in the presence of the antigenic peptide (Pep). (B) Kinase activity associated with cyclin E (○) or Cdk4 (◊) from multiple experiments was quantitated and graphed as percent control in the absence of added His6-p27. Points with error bars are representative of at least three independent experiments. (C) Cyclin E was immunoprecipitated from the lysates incubated with His6-p27 and from ORG 2058-treated cell lysate; the immune complexes were separated by SDS-PAGE and Western blotted with the indicated antibodies. The ratio of intensities of total p27Kip1 to cyclin E in the immunoprecipitate is shown.