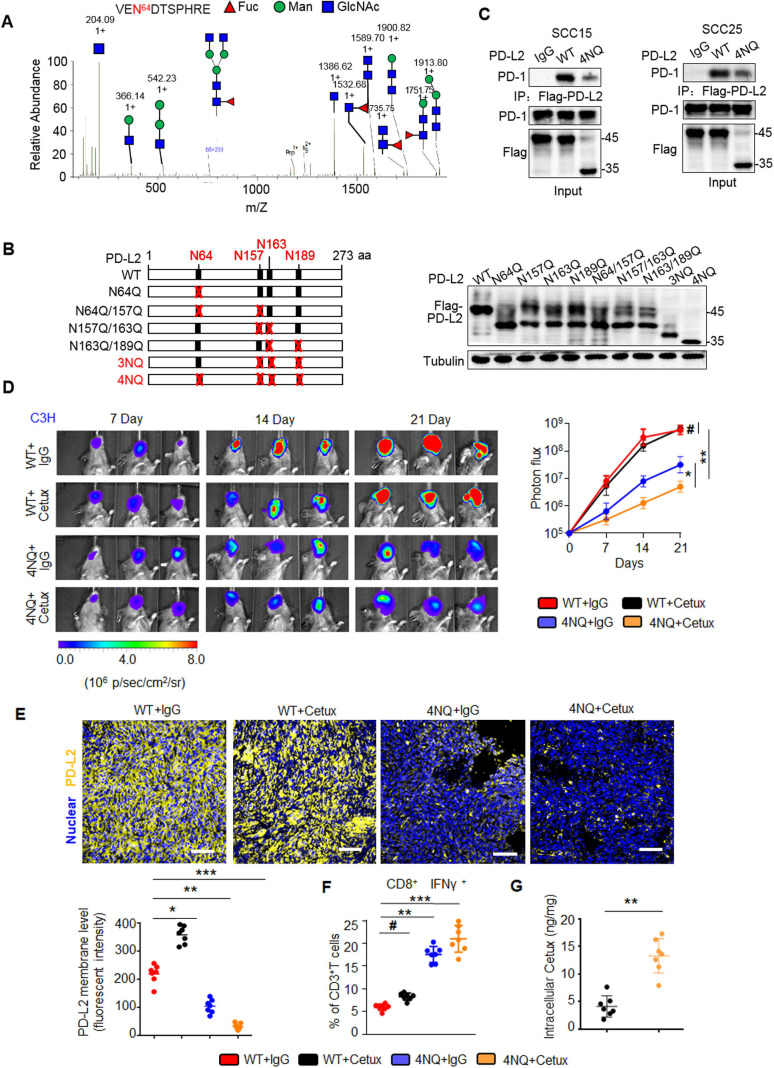
Figure 2.
PD-L2 glycosylation modulates antitumor immunity and the cetux response in HNSCC. (A) Identification of N-glycopeptides by LC-MS/MS corresponds to one of four N-glycosylation sites, namely, (N64). (B) Schematic representation of several PD-L2 NQ mutants used in this study. The four hypothetical NXT patterns are marked in red. The numbers indicate the position of aspartic acid. Protein levels of wild type (WT) or NQ mutants of PD-L2. (C) Co-immunoprecipitation (Co-IP) analysis of the interaction of PD-L2 WT or 4NQ mutants of PD-L2 with PD-1 in SCC15 and SCC25 cells. (D) Representative bioluminescence images of SCC7 cells expressing PD-L2 WT or 4NQ mutant in C3H mice treated with cetux every week (n=7 mice per group). Values represent mean±SD of the number of designated mice; *p<0.05; **p<0.01; #, not significant. (E) Immunofluorescence staining of PD-L2 in the tumor mass of mice with SCC7 tumors. Scale bar, 20 µm. Bottom panel depicts the quantification, *p<0.05; **p<0.01; ***p<0.001. (F) Quantification of IFNɣ in CD8+ and CD3+ T cell populations in C3H mice bearing SCC7 tumors using intracellular cytokine staining at day 21. **p<0.01; ***p<0.001; #, not significant. Error bars denote mean±SD of three independent experiments. (G) The intracellular cetux content in the tumor mass of mice with SCC7 tumors was detected using surface plasmon resonance. **p<0.01. HNSCC, head and neck squamous cell carcinoma; IFNγ, interferon gamma; LC-MS/MS, liquid chromatography tandem mass spectrometry; PD-L2, programmed death ligand 2.