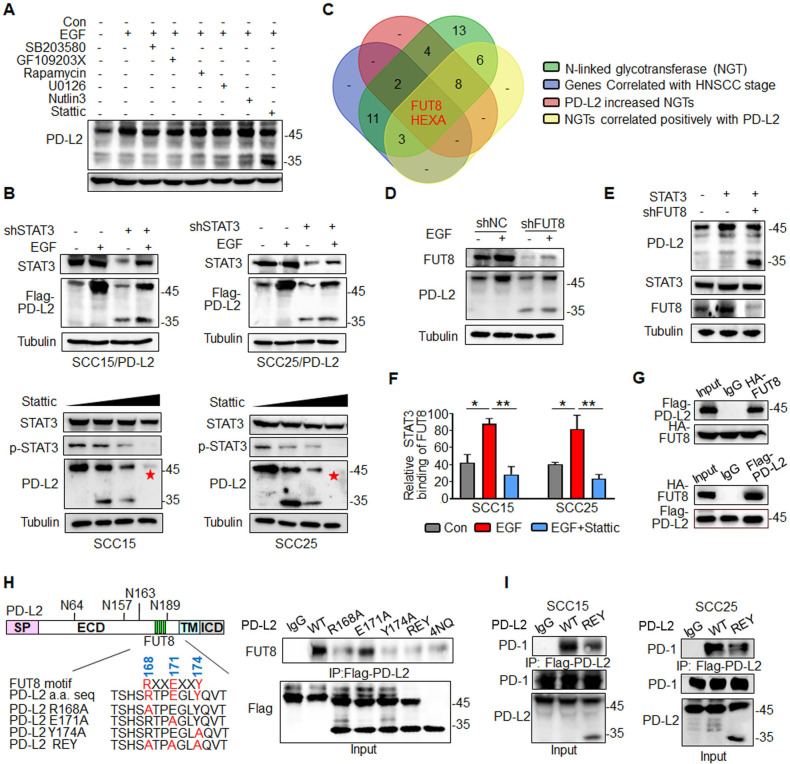
Figure 3.
EGF/STAT3 signaling promotes PD-L2 glycosylation through the N-glycosyltransferase FUT8. (A) PD-L2 protein levels in SCC15 cells pretreated with 7μM SB203580 (MAPK inhibitor), 7 µM GF109203X (PKCα inhibitor), 7 µM rapamycin (mTOR inhibitor), 7 µM U0126 (MEK1/2 inhibitor), 7 µM Nutlin-3 (MDM2 inhibitor) or 7 µM Stattic (STAT3 inhibitor) for 4 hours and subsequently incubated with EGF for 15 min.(B) PD-L2 protein levels in shSTAT3 knockdown clones were detected using an anti-Flag antibody (upper panels). SCC15 and SCC25 cells were treated with incremental concentrations of Stattic (7–14 µM) for 4 hours and the PD-L2 levels were analyzed (lower panels). (C) Identification of NGT that potentially regulates PD-L2 glycosylation. Venn diagram demonstrating the expression pattern of distinct NGTs in HNSCC. Two potential NGTs (FUT8 and HEXA) were identified. (D) PD-L2 protein levels in EGF-treated in SCC15 cells transfected with negative control or short hairpin RNA against FUT8. (E) PD-L2 expression levels in STAT3-overexpressing and FUT8 knockdown cells. (F) Chromatin immunoprecipitation assay results revealed that Stattic-mediated inhibition of STAT3 impaired EGF-induced binding of STAT3 to the promoter of FUT8. *p<0.05; **p<0.01. (G) Co-immunoprecipitation (Co-IP) was performed to measure the interaction of FUT8 with PD-L2 in SCC15 cells. (H) Schematic diagram of FUT8-binding motifs and several mutants of the PD-L2 expression structure. ECD, extracellular domain; ICD, intracellular domain; SP, signal peptide; TM, transmembrane domain. The numbers reveal amino acid positions. The binding affinity between various PD-L2 mutants and FUT8 was analyzed using Co-IP. (I) Co-IP analysis of the interaction of PD-1 with PD-L2 in SCC15 and SCC25 cells expressing wild type (WT) or REY PD-L2. EGF, epidermal growth factor; FUT8, fucosyltransferase; HNSCC, head and neck squamous cell carcinoma; PD-L2, programmed death ligand 2; REY. PD-L2 REY mutant, in which the amino acid of R168, E171 and Y174 were substituted by Alanine; STAT3, signal transducer and activator of transcription 3.