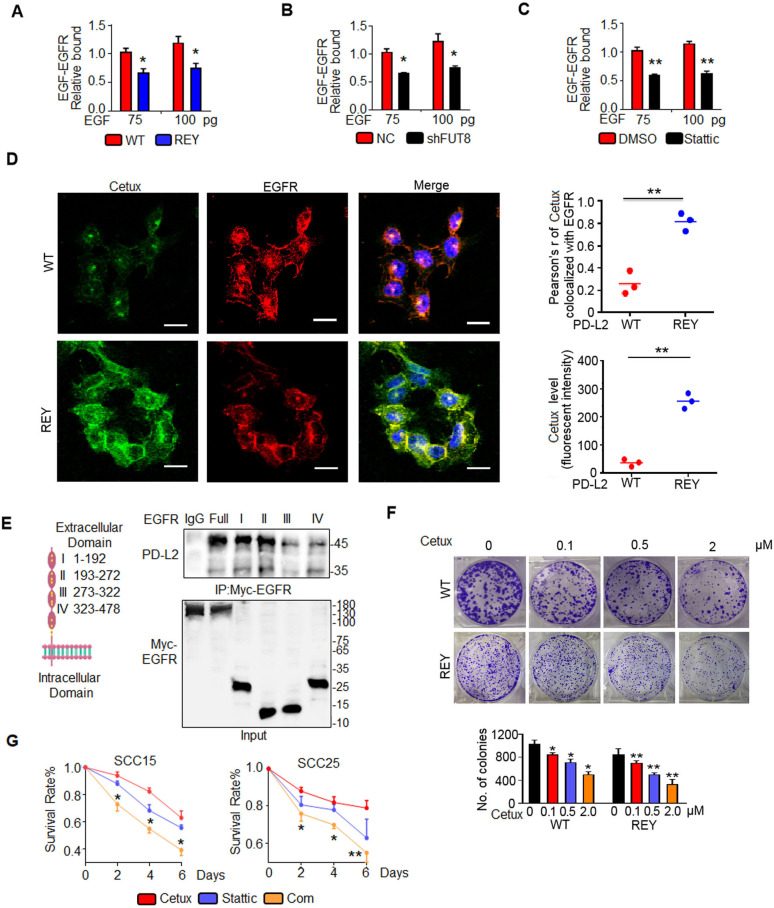
Figure 6.
Glycosylated PD-L2 enhances EGF-EGFR binding affinity. (A) EGF-EGFR binding affinity in SCC15 cells expressing PD-L2 WT or REY mutant was measured using capture ELISA. Significance was determined using one-way analysis of variance. *p<0.05. (B) Loss of FUT8 decreased the binding between EGF and EGFR in SCC15 cells. *p<0.05. (C) EGF-EGFR binding affinity in SCC15 cells treated with DMSO or Stattic was measured using capture ELISA. **p<0.01. (D) Confocal microscopy of SCC15 cells expressing PD-L2 WT or REY treated with EGF for 30 min and stained with anti-EGFR antibodies and FITC-labeled cetux. Scale bar, 20 µm. Right panel shows the quantification of the colocalization between cetux and EGFR. Quantification of the cetux fluorescence intensity in (D). Values are represented as mean±SD from three independent experiments. Significance was determined using one-way analysis of variance (ANOVA). **p<0.01. (E) Description and schematic representation of EGFR indicating specific sites mutated in individual Ig-like domains. Immunoblotting of EGFR and PD-L2 in total lysates of SCC15 cells transfected with EGFR-Myc-WT or EGFR-Myc-(IgI-IV) mutants. (F) Representative images of the colony formation capability of SCC15 cells. Significance was detected using repeated-measures ANOVA. *p<0.05; **p<0.01. (G) Quantification of viability of SCC15 and SCC25 cells treated with control, cetux, Stattic, or their combination (Com) for the indicated time. Error bars represent SD (n=3). *p<0.05; **p<0.01. EGFR, epidermal growth factor receptor; FUT8, fucosyltransferase; PD-L2, programmed death ligand 2; REY, PD-L2 REY mutant, in which the amino acid of R168, E171 and Y174 were substituted by Alanine; WT, wild type.