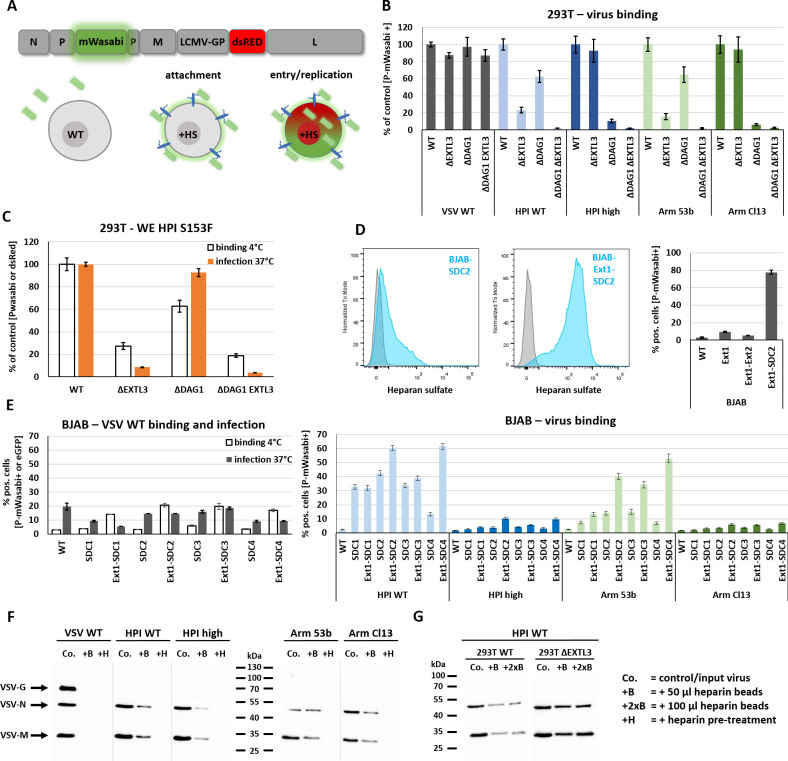
Fig 6. Proteoglycan expression restores HS expression on cell surface of BJAB cells and enables LCMV GP mediated virus attachment.
(A) Schematic overview of a directly labelled VSV-GP construct encoding a P-mWasabi fusion construct and dsRed on 5th position that allows to distinguish between attachment and virus entry/replication. Virus attachment was quantified by flow cytometry analysis. For attachment analysis, cells were incubated with directly labelled VSV-P-mWasabi-G or -GP variants for 30–45 min at 4°C in a total volume of 50 μl PBS or complete growth medium. Before measurement, samples were washed twice with PBS. Created with BioRender.com. (B) VSV-G and LCMV-GP mediated attachment of low or high affinity variants to 293T WT, ΔEXTL3, ΔDAG1, and ΔDAG1 EXTL3 cells. (C) Binding (4°C for 30 min) and infection (MOI 1, 37°C, 14 h p.i.) assay with 293T WT and variants for WE HPI S153F carrying the low affinity mutation described for WE2.2. (D) Flow cytometry analysis of HS expression in BJAB cells stably transduced with SDC2 or the combination of EXT1-SDC2 (left). GP-mediated (HPI WT) virus attachment to BJAB cells stably transduced with EXT1, EXT1-EXT2 or EXT1-SDC2 (right). (E) Different members of the syndecan family (SDC1–4) were tested alone or in combination with EXT1 in BJAB cells for their ability to enable VSV-G or LCMV-GP mediated attachment and infection. (F) In a pulldown assay, 50 μl heparin-coated agarose beads (+B) were incubated with purified VSV WT or VSV-GP variants at 4°C for 4 h under constant rotation. After four washing steps with PBS + 0.2% BSA, the beads were resuspended in 100 μl RIPA buffer, incubated for 20 min on ice and boiled at 95°C for 10 min after the addition of 4x SDS loading buffer. Western blotting was performed with anti-VSV serum (detecting VSV-G, -N, -M). co. = same amount of input virus used for incubation with heparin-coated agarose beads. (G) Bead-based pulldown assay performed with purified VSV-GP HPI WT produced on 293T WT or 293T ΔEXTL3 cells. Signal intensity was compared using 50 μl or 100 μl heparin-coated agarose beads to control for bead saturation effects. A pre-stained page ruler was used with the range of 10–180 kDa. Shown are the means ± SD of three replicates.