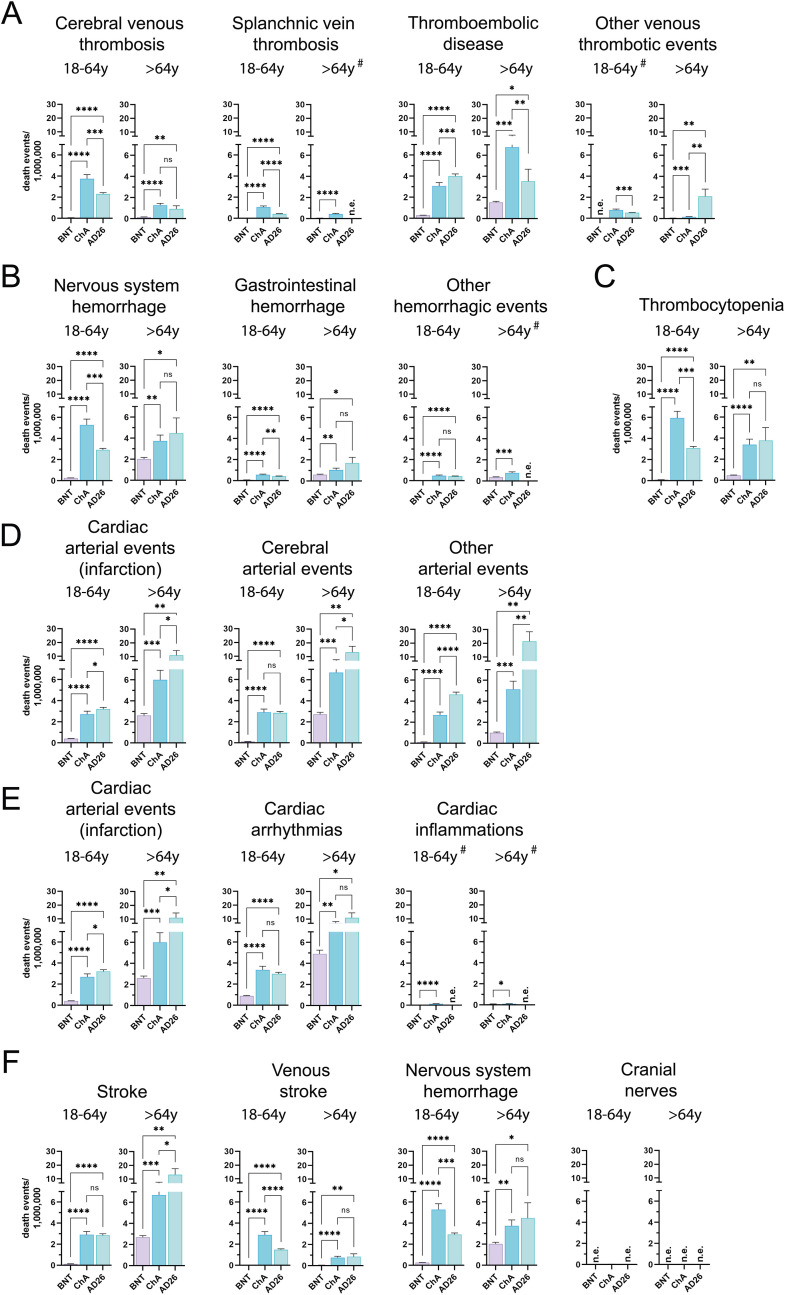
Fig. 4.
Frequency of individual cases with specific SAE-related death among BNT, ChA, and AD26 recipients divided according to age group. The frequency of individual cases with specific AEs divided according to age range (18–64 years and >64 years) was obtained by normalizing the number of individual cases with the doses supposedly administered to each age range in Europe at week 23 (Table S7). To do this, we considered the doses administered by the European countries providing data on the administration of each vaccine to these age ranges and established a method for evaluating the variance of the doses (see the Method section for details). The figure shows the frequency (mean ± SD) of individual cases with deaths related to SAEs consisting of (A) venous thrombotic SAEs (CVT, SVT, thromboembolic disease, other venous thrombotic events); (B) hemorrhagic SAEs (nervous system hemorrhage, gastrointestinal hemorrhage, other hemorrhagic events); (C) thrombocytopenia; (D) arterial SAEs (cardiac arterial events – myocardial infarction, cerebral arterial events, other arterial events); (E) cardiac SAEs (cardiac arterial events – myocardial infarction, arrhythmias, cardiac inflammation); myocardial infarction SAEs are also reported in (D); (F) nervous system SAEs (stroke, venous stroke, nervous system hemorrhage, cranial nerves); nervous system hemorrhage is also reported in (B). When deaths were observed for all three vaccines, significance was calculated using the Welch and Brown-Forsythe one-way ANOVA test; the difference between ChA and BNT, AD26 and BNT, and ChA and AD26 was assessed with Dunnett T3 multiple-comparison post-hoc analysis, and multiplicity-adjusted p-values are reported. When deaths were observed for only two vaccines (#), the unpaired t-test with Welch's correction was used. ns = p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.