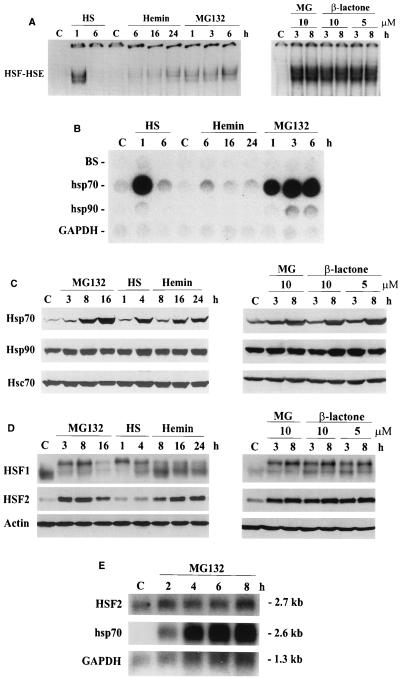
FIG. 1.
Heat shock response caused by inhibition of the ubiquitin-mediated proteolysis (A) HSF HSE-binding activity in whole cell extracts from control (C), heat-shocked (HS; 42°C for 1 and 6 h), hemin-treated (40 μM for 6, 16, and 24 h), MG132-treated (10 μM for 1, 3, 6, and 8 h), and clasto-lactacystin β-lactone-treated (10 or 5 μM for 3 and 8 h) K562 cells was analyzed by gel mobility shift assay. Extracts (12 μg) were incubated with a 32P-labeled oligonucleotide representing the proximal HSE of the human hsp70 promoter. Protein-DNA complexes were resolved on a 4% nondenaturing polyacrylamide gel as described elsewhere (26). (B) Transcription rates of hsp70 and hsp90 genes were analyzed by nuclear run-on assay. Equal number of nuclei from control (C), heat-shocked (HS; 1 and 6 h at 42°C), hemin-treated (40 μM for 6, 16, and 24 h), and MG132-treated (10 μM for 1, 3, and 6 h) K562 cells were used for in vitro [32P]dUTP labeling of newly synthesized transcripts which were hybridized to immobilized DNA probes for human hsp70, human hsp90/89α, rat GAPDH, and a Bluescript vector (BS) as described elsewhere (3). GAPDH was used as an internal control. (C and D) For protein analysis, whole cell extracts (12 μg) isolated from control (C), MG132-treated (10 μM for 3, 8, and 16 h), heat-shocked (HS; 42°C for 1 and 4 h), hemin-treated (40 μM for 8, 16, and 24 h), and clasto-lactacystin β-lactone-treated (10 μM for 3 and 8 h) K562 cells were analyzed by SDS-PAGE and Western immunoblotting using antibodies against HSF1, HSF2, Hsp70, Hsp90, Hsc70, and actin. (E) Poly(A) mRNA from control (C) and MG132-treated (10 μM for 2, 4, 6, and 8 h) K562 cells was analyzed by Northern blotting using 32P-labeled cDNA probes for human HSF2, hsp70, and GAPDH. GAPDH was used as a control for equal loading. mRNA sizes are indicated on the right.