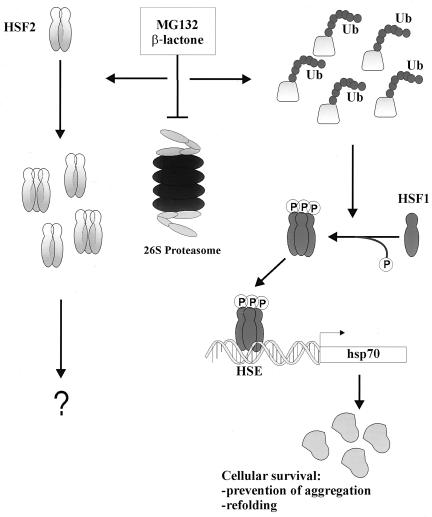
FIG. 4.
Model of HSF1-dependent Hsp70 induction in the ubiquitin (Ub)-proteasome pathway. Down-regulation of ubiquitin-mediated proteolysis by proteasome inhibitors, such as MG132 and clasto-lactacystin β-lactone, results in the accumulation of malfolded, abnormal, and short-lived proteins normally degraded by the 26S proteasome (6). Increase in the amount of polyubiquitinated proteins leads to oligomerization, phosphorylation, and acquisition of HSF1 HSE-binding activity, which in turn induces hsp70 transcription and accumulation of the Hsp70 protein, leading to enhanced molecular chaperone activity. It can be speculated that by associating with the polyubiquitinated proteins, molecular chaperones, among them Hsp70, promote the cellular survival by preventing protein aggregation and facilitating the malfolded proteins to refold. On the other hand, proteasome inhibition results in accumulation and partial oligomerization of the labile HSF2 protein, but the specific role of HSF2 under these conditions remains to be elucidated.