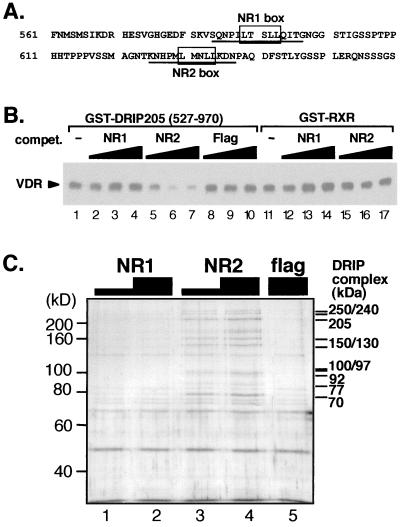
FIG. 4.
DRIP205-VDR interaction is selectively competed by the NR2 peptide. (A) Sequence of DRIP205 encompassing the two potential nuclear receptor-interacting motifs, NR1 and NR2. Underlined amino acids correspond to the sequences of NR1 and NR2 peptides used in subsequent experiments. (B) GST pull-down assay using 2 μg of GST-DRIP205 fragment (527 to 970) and purified VDR in the presence of 10−6 M 1,25(OH)2D3. NR1 (lanes 2 to 4) and NR2 (lanes 5 to 7) peptides (A) and an unrelated peptide (Flag; lanes 8 to 10) were used as competitors in 2-, 5-, and 20-fold molar excess over GST-DRIP205. The same amounts of NR peptides were coincubated with GST-RXR (lanes 11 to 17), here used as a control bait. (C) NR2 peptide is sufficient to elute the entire DRIP complex from VDR. GST-VDR LBD was used to pull down the DRIP complex from nuclear extracts as described previously (35). The complex immobilized on beads was then incubated with 5 or 30 μM NR1 or NR2 or 30 μM nonspecific (Flag) peptide. Bands matching subunits of the DRIP complex (34) are shown on the right.