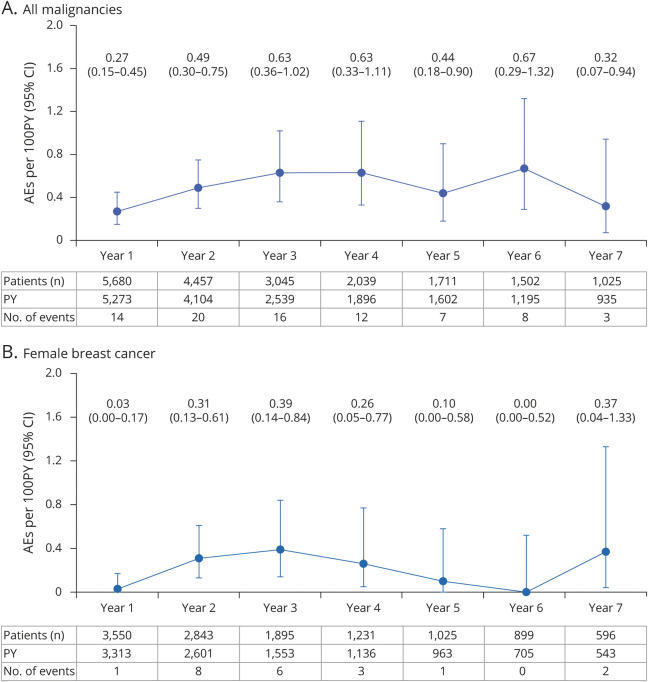
Figure 4. Yearly Crude Incidence Rates of all Malignancies and Female Breast Cancer in Ocrelizumab (OCR) All-Exposure Population.
Data cutoff: January 2020. Includes patients who received any dose of OCR during the controlled treatment period (CTP) and associated open-label extension (OLE) periods of the phase 2 and phase 3 studies, including patients originally randomized to comparator (interferon [IFN]-β-1a or placebo) who switched to open-label OCR treatment, plus VELOCE, CHORDS, CASTING, OBOE, ENSEMBLE, CONSONANCE, and LIBERTO. (A) Crude incidence rates of all malignancies from years 1–7, including nonmelanoma skin cancer (NMSC). Crude incidence rate of all malignancies (including NMSC) in the OCR all-exposure population, as of January 2020: 0.46 (0.37–0.57). (B) Crude incidence rates of female breast cancer from years 1–7. Data on yearly crude incidence rates of all malignancies and female breast cancer shown until year 7. Year 7 data are not mature due to limited exposure (543 patient-years [PY] for female breast cancer). AE = adverse event; CI = confidence interval.