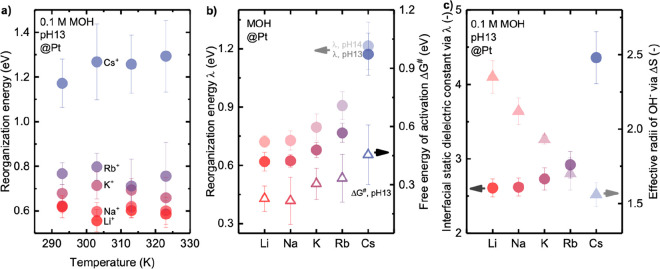
Figure 4.
Fitting temperature-dependent kinetics of HER/HOR on Pt RDE in alkaline electrolytes using the MHC formalism: (a) reorganization energy measured at pH 13 in 0.1 M hydroxide of Li+, Na+, K+, Rb+, and Cs+ for a temperature range from 293 to 323 K; (b) comparison of reorganization energy of HER/HOR at pH 13 and pH 14 by the MHC formalism, and the free energy of activation ΔG⧧ for HER/HOR at pH 13 obtained by Arrhenius analysis; (c) cation-dependent interfacial static dielectric constant of water extracted by Born model of reorganization energy and effective radii of OH– fitted using Born model of reaction entropy. Error bars were obtained from the standard deviation of 2–3 independent measurements. Full data points of reorganization energy at pH 13 are available in Figure S9.