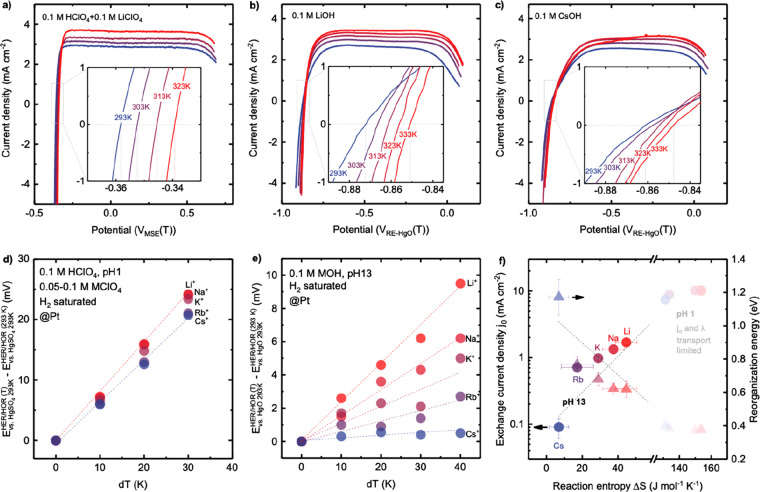
Figure 5.
Cation- and pH-dependent reaction entropy change of HER/HOR on
Pt RDE, determined by 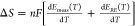 (Appendix S2). Temperature-dependent CV of HER/HOR measured on Pt at pH 1 in
H2-saturated 0.1 M HClO4 electrolyte (a) with
0.1 M LiClO4. Temperature-dependent CV of HER/HOR measured
on Pt at pH 13 of H2-saturated (b) 0.1 M LiOH and (c) 0.1
M CsOH. Insets show the zoom-in of zero overpotential region. Temperature-dependent
HER/HOR potential vs the potential of reference electrode (Hg/HgSO4 for pH 1 and Hg/HgO for pH 13) at 293 K (d) at pH 1 and (e)
at pH 13. (f) Cation dependence of the exchange current density j0 and reorganization energy, as a function of
reaction entropy, ΔS, of HER/HOR at pH 1 and
pH 13. Note that the kinetics (j0 and
λ) of HER/HOR on Pt RDE at pH 1 was limited by mass transport
(Figure S4) and cannot be separated from
diffusion current density, thus the exchange current density at pH
1 shown in Figure S5c does not reflect
the pure HER/HOR kinetics at pH 1. The thermodynamic HER/HOR entropy
changes were found to increase in the sequence of Cs+ <
Rb+ < K+ < Na+ < Li+, where adsorbed Cs+ and Rb+ could expel
interfacial water molecules and weaken the hydrogen-bonding network
of surrounding water molecules at OHL, which leads to an increase
in molar entropy of interfacial water molecules SH2O(l) and interfacial hydronium SH3O+(aq), and a corresponding decrease
in HER/HOR reaction entropy, ΔS = SH2(g) – 2 × SH3O+(aq) in acid (2H(aq)+ + 2e– ↔ H2(g)) or ΔS = SH2(g) + 2 × SOH–(aq)) – 2 × SH2O(l) in alkaline electrolytes (2H2O(l) + 2e– ↔
H2(g) + 2OH(aq)). Error bars were obtained
from the standard deviation of 2–3 independent measurements.
Full data points are available in Figure S10.
(Appendix S2). Temperature-dependent CV of HER/HOR measured on Pt at pH 1 in
H2-saturated 0.1 M HClO4 electrolyte (a) with
0.1 M LiClO4. Temperature-dependent CV of HER/HOR measured
on Pt at pH 13 of H2-saturated (b) 0.1 M LiOH and (c) 0.1
M CsOH. Insets show the zoom-in of zero overpotential region. Temperature-dependent
HER/HOR potential vs the potential of reference electrode (Hg/HgSO4 for pH 1 and Hg/HgO for pH 13) at 293 K (d) at pH 1 and (e)
at pH 13. (f) Cation dependence of the exchange current density j0 and reorganization energy, as a function of
reaction entropy, ΔS, of HER/HOR at pH 1 and
pH 13. Note that the kinetics (j0 and
λ) of HER/HOR on Pt RDE at pH 1 was limited by mass transport
(Figure S4) and cannot be separated from
diffusion current density, thus the exchange current density at pH
1 shown in Figure S5c does not reflect
the pure HER/HOR kinetics at pH 1. The thermodynamic HER/HOR entropy
changes were found to increase in the sequence of Cs+ <
Rb+ < K+ < Na+ < Li+, where adsorbed Cs+ and Rb+ could expel
interfacial water molecules and weaken the hydrogen-bonding network
of surrounding water molecules at OHL, which leads to an increase
in molar entropy of interfacial water molecules SH2O(l) and interfacial hydronium SH3O+(aq), and a corresponding decrease
in HER/HOR reaction entropy, ΔS = SH2(g) – 2 × SH3O+(aq) in acid (2H(aq)+ + 2e– ↔ H2(g)) or ΔS = SH2(g) + 2 × SOH–(aq)) – 2 × SH2O(l) in alkaline electrolytes (2H2O(l) + 2e– ↔
H2(g) + 2OH(aq)). Error bars were obtained
from the standard deviation of 2–3 independent measurements.
Full data points are available in Figure S10.