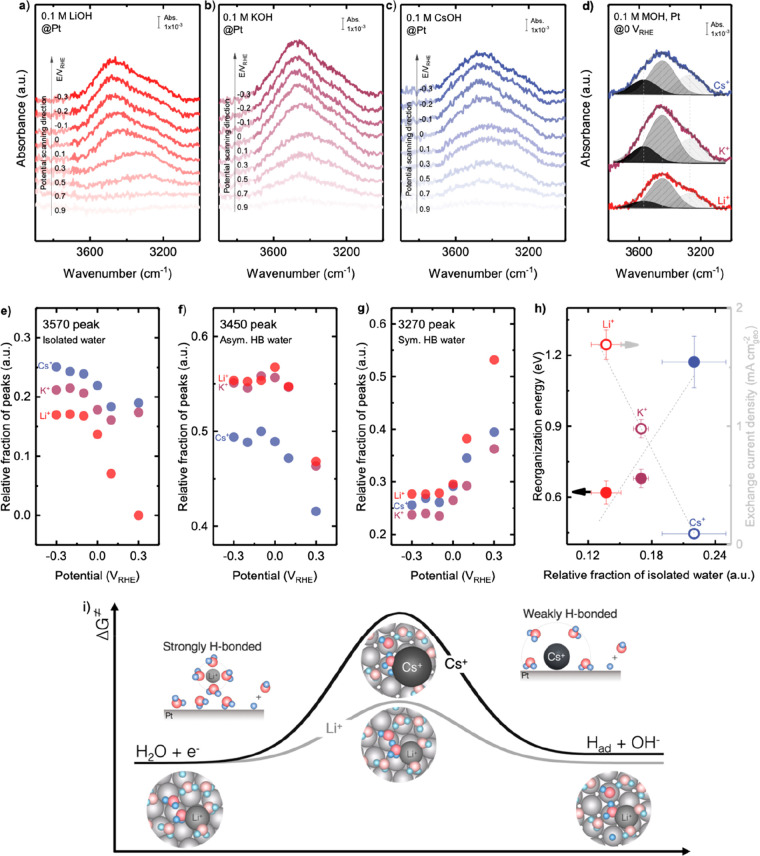
Figure 6.
Altering interfacial structure at the Pt surface in aqueous electrolytes through noncovalent interactions associated with the hydrated cations, water molecules, and negatively charged Pt surface. The potential-dependent OH stretching features of in situ SEIRAS spectra of HER/HOR measured from 0.9 VRHE to −0.3 VRHE in a H2-saturated aqueous solution of 0.1 M of (a) LiOH, (b) KOH, and (c) CsOH, where the reference spectrum was taken at 1.1 VRHE. Full spectra before and after subtracting by reference spectrum (at 1.1 VRHE) are available in Figure S11. (d) Deconvolution of the OH stretching peak at 0 VRHE into three components: 3570 cm–1 (weakly H-bonded/isolated water), 3450 cm–1 (asymmetric H-bonded water), and 3270 cm–1 (symmetric H-bonded water). The potential dependence of the relative fractions of (e) isolated water, (f) asymmetric H-bonded water, and (g) symmetric H-bonded water. (h) Reorganization energy and exchange current density of HER/HOR measured on Pt RDE in 0.1 M hydroxide of Li+, K+, and Cs+ as a function of the relative fraction of isolated water. Error bars were obtained from the standard deviation of 2 independent measurements. (i) Scheme summarizing the proposed mechanisms of cation dependent kinetics of HER/HOR, where Li+ ions retain their solvation shell intact and promote strongly hydrogen-bonded interfacial water layer, enhance proton-coupled electron transfer rate of the Volmer or Heyrovsky step by facilitating interfacial water molecule reorganization. Cs+ ions partially desolvate and attach to the negatively charged Pt surface, promoting weakly H-bonded interfacial water molecules, leading to an increase of the barrier of proton transfer during the Volmer or Heyrovsky step in HER/HOR on the Pt surface.