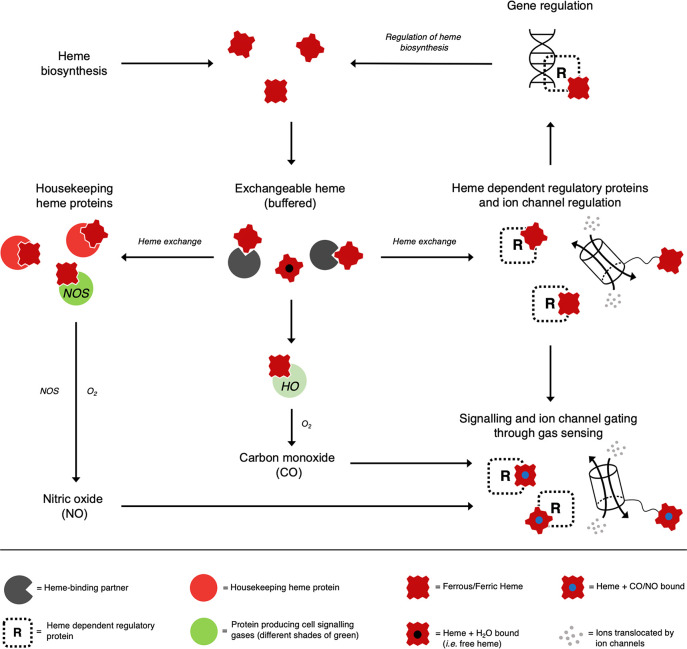
Figure 2.
The possible interconnected pathways for the movements of heme in cells, and the links to signaling gases such as CO and NO. From the total heme synthesized in the cell (top), a proportion is bound irreversibly to heme-binding housekeeping proteins (red circles) that are essential for cell survival. A body of exchangeable heme is envisaged as being mostly weakly bound, soon after heme biosynthesis, to heme-binding partners (dark gray pacmans in this and other figures, which could be heme proteins or non-heme proteins) and available for exchange to heme dependent regulatory proteins (R, right). These heme-binding partners constitute an exchangeable, buffered, reservoir that can provide a flexible supply of heme and protect against changes in heme concentration. Once formed, these heme-bound proteins can serve in regulatory roles by, for example, binding to DNA for transcriptional control (top right; including the regulation of heme biosynthesis17,21,28,37,192−194) or to ion channels (middle and bottom right54,195). In green circles are shown the proteins that produce cell signaling gases—nitric oxide synthase (NOS, left) and heme oxygenase (NOS, middle). The synthesis of NO by NOS, and the production of CO by the heme degrading HO enzyme, adds multiple layers of complexity by coupling the formation of cell signaling gases to the heme-binding process. This would allow both CO and NO to bind to any heme protein with a regulatory function (bottom right) but could equally well occur for other heme dependent regulatory processes. It is worth noting that, while the binding of π-acid ligands like CO is traditionally associated exclusively with heme in its ferrous form, ferric heme has also been shown capable of binding CO/NO.41 For the purposes of this review, movement of ferric/ferrous heme is presumed to mean heme b.