Abstract
A novel bacterium, designated strain Msb3T, was recently isolated from leaves of the yam family plant Dioscorea bulbifera (Dioscoreaceae). Phylogenetic analysis based on the 16S rRNA gene sequence indicated that this strain belonged to the genus Paraburkholderia with Paraburkholderia xenovorans as nearest validly named neighbour taxon (99.3 % sequence similarity towards the P. xenovorans type strain). Earlier genome sequence analysis revealed a genome of 8.35 Mb in size with a G+C content of 62.5 mol%, which was distributed over two chromosomes and three plasmids. Here, we confirm that strain Msb3T represents a novel Paraburkholderia species. In silico DNA–DNA hybridization and average nucleotide identity (OrthoANIu) analyses towards P. xenovorans LB400T yielded 58.4 % dDDH and 94.5 % orthoANIu. Phenotypic and metabolic characterization revealed growth at 15 °C on tryptic soy agar, growth in the presence of 1 % NaCl and the lack of assimilation of phenylacetic acid as distinctive features. Together, these data demonstrate that strain Msb3T represents a novel species of the genus Paraburkholderia, for which we propose the name Paraburkholderia dioscoreae sp. nov. The type strain is Msb3T (=LMG 31881T, DSM 111632T, CECT 30342T).
Keywords: Burkholderiaceae, Dioscorea, Paraburkholderia, plant growth promotion, tomato, yam, plant microbe interaction
Introduction
The genus Paraburkholderia contains species formally classified as Burkholderia that were separated from the latter on the basis of phylogenomic evidence [1]. The majority of Paraburkholderia species have been isolated from soil and plant roots or nodules but some have been found in aquatic environments and as opportunistic human pathogens, too [2–7]. Bacteria within the genus usually carry large genomes (7–10 Mb) and are metabolically versatile. Notable is the ability of some to nodulate legumes and fix atmospheric nitrogen [8]. Their tendency to occur in acidic soils [9–14] and the capacity to degrade tannins and phenolics as well as polyaromatic hydrocarbons and halogenated phenols [11, 12, 15–19] highlights their role in the decomposition of plant-derived aromatics and their putative role for bioremediation of polluted soils. Many isolates from the rhizosphere display plant growth promoting properties indicating their possible role as biofertilizers [20–24].
The present study was performed to formally classify strain Msb3T, which was isolated from the phyllosphere of the ‘air potato yam’, Dioscorea bulbifera [6]. Herpell et al. [6] demonstrated that this strain is closely related to Paraburkholderia xenovorans and reported several genomic, taxonomic, functional and ecological characteristics of this organism. Its genome encodes an impressive combination of features mediating a beneficial plant-associated lifestyle that include biological nitrogen fixation, plant hormone regulation, detoxification of xenobiotics, degradation of aromatic compounds and multiple protein secretion systems [6]. When applied to tomato, strain Msb3T exhibited significant growth promotion by increasing the total dry biomass by up to 40 % [6]. Below, we reiterate basic taxonomic information from Herpell et al. [6] and provide comparative phylogenomic, chemotaxonomic and phenotypic analyses to formally classify this strain as a novel Paraburkholderia species.
Isolation and ecology
Strain Msb3T was isolated from fresh Dioscorea bulbifera (yam family, Dioscoreaceae) leaves that were rinsed with water and surface-sterilized [6]. Leaf acumens and sections were ground in a 0.4 % sodium chloride solution and serial dilutions were plated on tryptic soy agar medium (TSA) and incubated aerobically at room temperature (ca. 22 °C) in the dark for 48 h. Nearly pure cultures of a single strain were obtained from every leaf acumen extract in several replicate experiments over several years, but not from leaf extracts. Single bacterial colonies were transferred and purified onto TSA agar plates. Bacteria were stored in 30 % glycerol in tryptic soy broth at −80 °C after initial identification. Additional details about the isolation are provided in a study describing the plant growth-promoting activity of strain Msb3T [6]. The culture was identified as a Paraburkholderia strain by comparative 16S rRNA and gyrB gene sequence analyses. It was designated Msb3T [6]. The ‘main’ 16S rRNA gene sequence extracted from the Msb3T genome (see Genome Features) exhibited 99.3 % sequence similarity with that of P. xenovorans LB400T and less than 98.8 % towards the type strains of other validly named Paraburkholderia species. The 16S rRNA gene-based amplicon sequencing earlier revealed that this organism was a predominant member of the D. bulbifera phyllosphere, making up to 25 % of the microbial community on leaf acumens [6]. The association was stable over time and reoccurred after completion of the plants’ growth cycles, indicating a long-term symbiotic relationship.
Genome features
The whole-genome sequence of strain Msb3T was determined previously using a PacBio Sequel instrument (Pacific Biosciences of California, Inc., Menlo Park, CA, United States) [6]. The Msb3T genome has a cumulative size of 8.35 Mbp and harbours 8199 coding sequences that are distributed over two chromosomes and three plasmids. The G+C content is 62.5 mol%. An in-depth characterization of functional gene content and phylogenetic analyses have been provided in reference [6]. Additional evidence for the delineation of a new species [25] is presented below.
It should be noted that six rrn operons were present in the genome sequence of strain Msb3T. They could be divided into two groups separated by a single SNP at position 1008 (T → C). The three identical copies on chromosome 1 were used to calculate the sequence similarity values presented above. This ‘multiple copy number issue’ was also found in other Paraburkholderia genomes [12] and should be recognized more widely. It adds to a line of evidence that the 16S rRNA gene has rather low discriminatory power and limited applicability as a phylogenetic marker for Paraburkholderia [12, 26].
Phylogenomic analyses
As shown previously [6], Burkholderia sp. Ch1-1, a polycyclic aromatic hydrocarbon (PAH) degrading soil isolate [27], is the closest relative of Msb3T. The genomes of strains Msb3T and Ch1-1 were uploaded to the Type (Strain) Genome Server (TYGS) [28] for a whole genome-based taxonomic analysis consisting of the determination of closely related type strains and the calculation of the pairwise digital DNA–DNA hybridization (dDDH) values and their confidence intervals using the recommended settings of the Genome-to-Genome Distance Calculator 2.1 [29]. The resulting dataset from TYGS, comprising the genomes of Msb3T, Ch1-1 and 15 closely related type strains (Table S1, available in the online version of this article), was also used to calculate the average nucleotide identity (ANI) values using the OrthoANIu algorithm [30]. Finally, whole-genome phylogeny was assessed based on 107 single-copy core genes found in a majority of bacteria [31] using bcgTree [32]. Visualization and annotation of the phylogenetic tree was performed using iTOL [33].
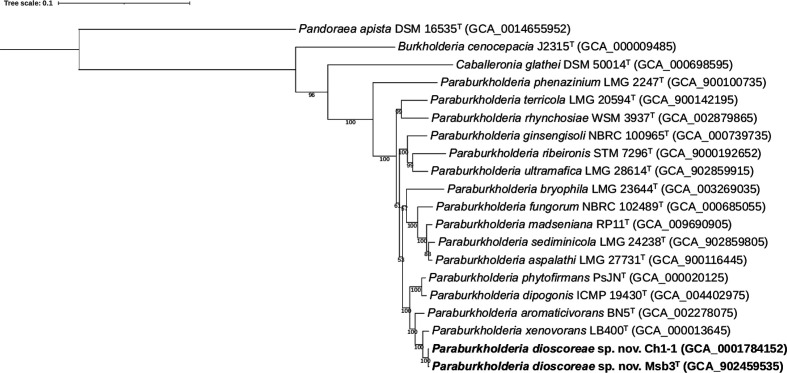
The phylogenomic tree was well resolved and showed high bootstrap support on most branches (Fig. 1). In congruence with earlier results [6], the present phylogenomic analysis showed that strain Ch1-1, P. xenovorans LB400T and Paraburkholderia aromaticivorans BN5T were the closest phylogenetic neighbour strains of strain Msb3T. Both orthoANIu and dDDH values confirmed that Burkholderia sp. Ch1-1 represents the same species as strain Msb3T as it shared dDDH and orthoANIu values of 96.5 and 99.5 %, respectively (Table 1). P. xenovorans LB400T shared dDDH and orthoANIu values of 58.4 and 94.5 %, respectively. All other Paraburkholderia species yielded dDDH and orthoANIu values well below the species delineation thresholds of 70 % [29] and 95–96 % [30], respectively (Table 1). Altogether, these genomic analyses confirmed that strain Msb3T represents a novel Paraburkholderia species with P. xenovorans as nearest neighbour taxon.
Fig. 1.
Phylogenetic tree based on 107 single-copy core genes. BcgTree was used to extract the nucleotide sequence of 107 single-copy core genes and to construct their phylogeny by partitioned maximum-likelihood analysis. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. Pandoraea apista DSM 16535T was used as outgroup. Bar, 0.1 changes per nucleotide position.
Table 1.
Pairwise dDDH and orthoANIu values between strain Msb3T and its nearest phylogenetic neighbours
|
Taxon |
dDDH (%) |
ANI (%) |
|---|---|---|
|
Paraburkholderia dioscoreae sp. nov. Ch1-1 |
96.5 |
99.5 |
|
Paraburkholderia xenovorans LB400T |
58.4 |
94.5 |
|
41.8 |
90.6 |
|
|
37.0 |
88.8 |
|
|
Paraburkholderia dipogonis ICMP 19430T |
37.1 |
88.6 |
Comparative genomic analysis between strains Msb3T and CH1-1
As the closest relative of strain Msb3T and, according to our in-silico analysis, the same species, we sought to include strain Ch1-1 in our polyphasic comparative genomics analysis. In light of the different source of isolation of strain Ch1-1, namely from PAH contaminated soil from sites of former coal gasification plants in Wisconsin [27], it may exhibit very interesting adaptations and high potential for bioremediation. The strain is, however, not available at any commercial strain collection, nor were we able to obtain the isolate through channels of private communication. We therefore decided to include a thorough comparison of the strain’s genome with that of strain Msb3T outlining similarities and differences between the two strains.
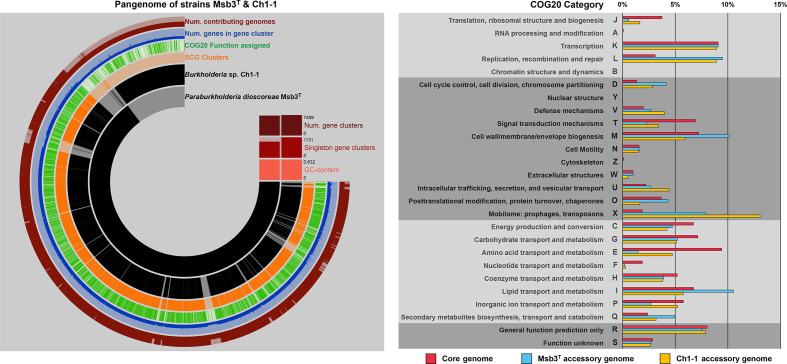
Making use of ANVIO’s pangenomics suite [34] all protein coding sequences of both genomes were clustered into gene clusters via NCBI’s blastp algorithm and using 10 as the MCL inflation parameter. For Msb3T and Ch1-1, 7465 and 7928 CDSs, respectively, could be used to compute the pangenome of both strains (Fig. 2). Both strains shared 6383 gene clusters comprised of 6549 and 6794 CDSs, respectively. A total of 890 CDSs in 919 clusters from the Msb3T genome and 1258 CDSs in 1131 clusters from the Ch1-1 genome comprised the accessory genomes of the two strains, accounting for 12.3 and 15.9 % of all CDSs, respectively. We analysed the accessory genomes in respect to their functionality. All accessory genes were classified into clusters of orthologous groups of proteins (COGs) [35] and relative differences in COG classification profiles were used to reflect on strain specific biases (Fig. 2). A large part of both accessory genomes consists of CDSs that are either not classifiable or have unknown functions (COG categories R and S), making up 52.8 and 48.4 % of the accessory genomes of strain Msb3T and Ch1-1, respectively. When considering only classifiable sequences, the enrichment of COG categories L (replication, recombination and repair) and D (cell cycle control, cell division, chromosome partitioning) in the accessory genomes of both strains is noteworthy. It could be explained through the presence of unique plasmids. At least in strain Msb3T, whose genome is closed, all three plasmids display a complete lack of synteny conservation to the genome of strain Ch1-1.
Fig. 2.
Pangenome analysis of strains Msb3T and Ch1-1. The left panel displays the pangenome of both strains created through ANVIO’s pangenomics suite [34]. CDSs from strain Msb3T and strain Ch1-1, respectively, are shown on ring 1 and 2 (starting inside). Presence of a CDS in either genome is indicated in black and absence in light grey. CDSs are forced into synteny with the genome sequence of strain Msb3T. Ring 3 displays whether two orthologs fall into self-consistency-grouping (SCG) clusters, ring 4, whether a COG20 function could be assigned to the CDS in the cluster. Ring 5 displays the number of genes that fall into a respective gene cluster, ranging from a minimum of one to a maximum of five, in form of a bar chart. The outermost ring, ring 6, indicates, how many genomes contribute to a gene cluster. If two genomes (full bar) contribute to a cluster, it was considered part of the core genome of the two strains. If, however, only one genome contributes (half bar), the respective CDS is considered part of that genome’s accessory genome (AG). The panel on the right shows the relative COG category classification patterns of the core genome of both strains in pink, the AG of strain Msb3T in light blue as well as the AG of strain Ch1-1 in yellow. The functional annotation into COG categories was conducted through the software package ANVIO, which implements the latest update of the COG database, COG20 [35]. Depicted is the percentage of all classifiable CDSs that fall into a given COG category.
The accessory genome of strain Ch1-1 is enriched in category U (intracellular trafficking, secretion, and vesicular transport) with a large number of components of the type IV secretory pathway present. And while the presence of mobile elements is notably high in both accessory genomes, the number of genes that belong to COG category X (mobilome: prophages, transposons) is exceptionally high in strain Ch1-1 making up more than 13 % of classifiable CDSs, compared to 7.87 % in strain Msb3T.
The accessory genome of strain Msb3T, on the other hand, is enriched in CDSs that belong to categories I (lipid transport and metabolism) and M (cell wall/membrane/envelope biogenesis) as well as Q (secondary metabolites biosynthesis, transport and catabolism). Interestingly, many CDSs classified within the latter are located on a genomic island (GI) on chromosome II. The GI is approximately 200 Kbp in length and 83 % of its CDSs are not in synteny with the genome of strain Ch1-1. Some of these CDSs share high similarity with the ferredoxin rhodocoxin and the associated gene cluster needed for the degradation of thiocarbamate herbicides via cytochrome P450 [36], a trait which may be useful to explore in a biotechnological context considering the potential of microbial degradation of agrochemicals in the root zone by plant associated bacteria.
Similarly, the phn gene cluster in strain Ch1-1, responsible for its capacity to degrade phenanthrene [27, 37], is located on a GI in scaffold 5 of the draft genome assembly and represents another interesting strain specific adaptation.
We also annotated the accessory genomes of both strains via the KEGG database [38] to spot pathways not indicated through a bias in COG category enrichment. Interestingly, strain Ch1-1 carries orthologs of cciI and cciR, responsible for N-acylhomoserine lactone (AHL) synthesis and its response regulator gene. These genes are usually found in species of the Burkholderia cepacia complex (Bcc) on a GI designated cenocepacia island [39] and affect their virulence and capacity to form biofilms [40, 41]. Both strains carry several unique genes connected to plant-microbe-interactions: while a gene encoding the flg22 epitope (fliC) is present in strain Msb3T but not in strain Ch1-1, an ortholog to the rice Xa21-immune response elicitor raxA is present in strain Ch1-1. However, most adaptations to a plant associated lifestyle are shared between both strains.
The two strains also display several interesting adaptations towards degradation of various phenolics. While both strains have the ability to degrade benzoate via catechol to acetyl-coA, only strain Ch1-1 has the capacity to completely degrade hydroxyquinol, 1,2,4-benzenetriol, nitrobenzene and 2-bromo-maleylacetate. Strian Ch1-1 also carries genes from the bph gene cluster to catalyse the degradation of polychlorinated biphenyl (PCB) and biphenyl itself. Both strains, however, lack the gene bphD, the 2,6-dioxo-6-phenylhexa-3-enoate hydrolase, necessary to complete PCB degradation to benzoate. Strain Ch1-1 certainly has a larger variety of genes encoding enzymes that take part in degradation of PAHs and other phenolic compounds, although many pathways are not complete.
Overall, the two genomes share a very high degree of similarity, indicative of a very close phylogenetic relationship. While the accessory genomes of both strains seem to be shaped by their respective habitat, leading to differences in metabolic potential, the general array of functions predicted on the basis of their genome seem to overlap (Fig. 2). We therefore propose that strain Ch1-1 is a different strain of the same species as strain Msb3T, as supported by dDDH and orthoANIu values (Table 1). We refrain, however, from describing it to the same degree of detail as strain Msb3T, seeing as we could not get a hold of a culture to include it in chemotaxonomic and physiological analyses.
Physiology and chemotaxonomy
A physiological characterization of strain Msb3T was performed earlier [6] and is presented below as part of the species description. An additional phenotypic characterization of strain Msb3T and of the type strains of its two nearest neighbour taxa, i.e. P. xenovorans LMG 21463T and P. aromaticivorans LMG 31771T was performed in the present study. All growth tests were recorded after 2, 3, 5 and 7 days of incubation; but test results after 2 days of incubation are presented below unless specified otherwise.
Cell and colony morphology were assessed after 3 days of incubation on phosphate buffered (0.45 g l−1 KH2PO4/2.39 g l−1 Na2HPO4.12H2O) nutrient agar [42] (Oxoid) at 28 °C. Motility was observed in young cultures by examining wet mounts in broth by phase-contrast microscopy. The assessment of catalase and oxidase activities and other tests were performed using conventional procedures [43]. Aerobic growth was tested at 28 °C on NAB, TSA, MacConkey agar (Oxoid), Drigalski agar (Biorad) and Pseudomonas cetrimide agar (Oxoid). The temperature growth range was tested on NAB and TSA at 4, 15, 20, 28, 37, 40 and 45 °C. The effect of NaCl on growth was investigated in nutrient broth (Oxoid) with different concentrations of NaCl (0–10 % with 1 % intervals, w/v). The pH range for growth was evaluated in nutrient broth buffered at pH 4.0 to 9.0 at intervals of 1 pH unit using the following buffer systems: acetate buffer (4.0–5.0), phosphate buffer (pH 6.0–8.0) and Tris-HCl (pH 9.0). Anaerobic growth was tested on NAB, TSA and TSA supplemented with 10 mM KNO3. Hemolysis of horse blood was tested using TSA supplemented with 5 % horse blood. DNase activity was tested using DNase agar (Sigma-Aldrich). Starch hydrolysis was tested using NAB and TSA supplemented with 0.8 % (w/v) soluble starch. Hydrolysis of casein was tested using Plate count agar (Oxoid) supplemented with 1.3 % (w/v) dried skim milk (Oxoid). Hydrolysis of Tween 20, 40, 60 and 80 was tested as described by Sierra (1957). The activity of constitutive enzymes and other physiological properties were determined after growth on TSA for 2 days at 28 °C using the API 20NE and API ZYM microtest systems (BioMérieux), according to the manufacturer’s instructions. Results were read after 24 h and 48 h, and after 4 h of incubation, respectively.
The three strains shared the following characteristics: growth on MacConkey and Drigalski agar and on Tween 20, 40, 60 and 80 agar base. Growth in the presence of 0 % NaCl and at pH 6 and 7. Growth at 20 and 28 °C on TSA and at 15, 20 and 28 °C on NAB. Hemolysis on horse blood agar after 5 days of incubation. Activities of the following enzymes were present: aesculin hydrolysis, assimilation of d-glucose, d-mannose, d-mannitol, N-acetyl-glucosamine, potassium gluconate, adipic acid, malate and trisodium citrate (API 20NE), and butyrate esterase (C4) (weak), caprylate esterase lipase (C8) (weak), alkaline and acid phosphatase, phosphoamidase and leucyl arylamidase (API ZYM).
The following characteristics were uniformly absent: growth on Pseudomonas cetrimide agar, hemolysis on horse blood agar after 2 and 3 days of incubation. DNase activity, hydrolysis of starch, casein and Tween 20, 40, 60 and 80. Anaerobic growth on NAB, TSA or TSA supplemented with 10 mM KNO3. Growth in the presence of 2–10% NaCl and at pH 4.0, 5.0, 8.0 and 9.0. Growth at 4, 37, 40 and 45 °C on TSA and growth at 4, 40 and 45 °C on NAB. When examined using the API 20NE microtest system, the following characteristics were absent: nitrate reduction, indol production from l-tryptophane, fermentation of glucose, activity of arginine dihydrolase and urease, gelatin liquefaction and assimilation of maltose. When examined using the API ZYM microtest system, the following enzyme activities were absent: myristate lipase (C14), valine arylamidase, cystin arylamidase, trypsin, chymotrypsin, alpha-galactosidase, beta-galactosidase, beta-glucuronidase, alpha-glucosidase, beta-glucosidase, N-acetyl-beta-glucosaminidase, alpha-mannosidase and alpha-fucosidase.
Table 2 presents biochemical characteristics that allow to differentiate strain Msb3T from P. xenovorans LMG 21463T and P. aromaticivorans LMG 31771T. Growth at 15 °C on TSA, growth in the presence of 1 % NaCl and the lack of assimilation of phenylacetic acid allowed to distinguish strain Msb3T from the former; catalase and oxidase activity, the assimilation of capric acid and the lack of assimilation of phenylacetic acid allowed to distinguish strain Msb3T from the latter.
Table 2.
Differential phenotypic characteristics of strain LMG 31881T and closely related type strains. All data are from the present study. +, Positive reaction; W, weakly positive reaction; −, negative reaction; (+), delayed positive
|
Characteristic |
P. dioscoreae |
||
|---|---|---|---|
|
LMG 31881T (Msb3T) |
LMG 21463T |
LMG 31771T |
|
|
Growth at: |
|||
|
15 °C on TSA |
+ |
− |
+ |
|
37 °C on NAB |
w |
+ |
w |
|
Growth in the presence of 1 % NaCl |
+ |
− |
+ |
|
Activity of: |
|||
|
Catalase |
+ |
+ |
(+) |
|
Oxidase |
+ |
+ |
w |
|
Activity of (API 20NE): beta-Galactosidase |
− |
− |
+ |
|
Assimilation of (API 20NE): |
|||
|
l-Arabinose |
w |
− |
+ |
|
Capric acid |
+ |
+ |
− |
|
Phenylacetic acid |
− |
+ |
+ |
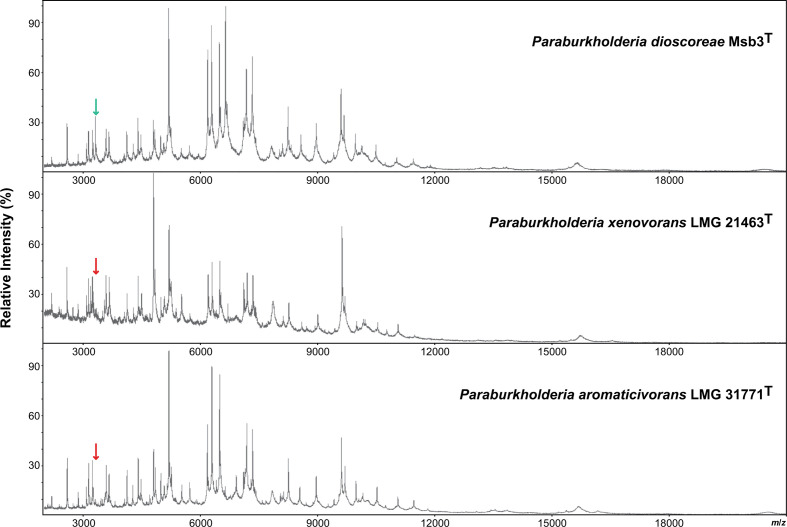
Finally, MALDI-TOF MS profiles of strains Msb3T from P. xenovorans LMG 21463T and P. aromaticivorans LMG 31771T were obtained as described before [44]. All strains were cultivated on NAB at 28 °C for 2 days before cell extracts were prepared. Strains Msb3T and P. xenovorans LMG 21463T had highly similar MALDI-TOF MS profiles but could be differentiated by a peak at m/z 3321.8 in the former profile. P. aromaticivorans LMG 31771T had a clearly distinct MALDI-TOF MS profile (Fig. 3). Today, MALDI-TOF MS is used worldwide as the leading diagnostic instrument for species level identification of medical, pharmaceutical and food-related bacteria [45, 46] and its use for studying environmental ecosystems is increasing rapidly [47]. The data produced in the present study demonstrated that MALDI-TOF MS reference libraries, supplemented with reference spectra of the Paraburkholderia species studied, will facilitate species level identification. Unlike other chemotaxonomic markers routinely included in polyphasic taxonomic studies today [48] this provides practical diagnostic value.
Fig. 3.
MALDI-TOF MS profiles of strains Msb3T, P. xenovorans LMG 21463T and P. aromaticivorans LMG 31771T. Peaks were visualized by mMass v. 5.5.0 [49]. The peak at m/z 3321.8 allows to distinguish strain Msb3T from P. xenovorans LMG 21463T, as indicated by the green (present) and red (absent) arrows.
In conclusion, the present phylogenomic (Figs 1 and 2, Table 1) and phenotypic (Table 2, Fig. 3) analyses confirmed that strains Msb3T and Ch1-1 represent a novel Paraburkholderia species that can be distinguished genotypically from the type strains of P. xenovorans and P. aromaticivorans, its nearest phylogenetic neighbour taxa. Additionally, chemotaxonomic and phenotypic analyses of the type strain, Msb3T, could confirm this distinction. We therefore propose to formally classify this strain into the novel species Paraburkholderia dioscoreae sp. nov., with Msb3T (=LMG 31881T, DSM 111632T, CECT 30342T) as the type strain.
Full-Text
Description of Paraburkholderia dioscoreae sp. nov.
Paraburkholderia dioscoreae sp. nov. (Di.os.co.re’ae. N.L. gen. n. Dioscoreae, of Dioscorea, referring to the plant genus source of isolation).
Cells are Gram-stain-negative, motile and rod-shaped. Optimal growth of strain Msb3T is observed between 22 and 28 °C in aerobic conditions. After 3 days of incubation on NAB at 28 °C, colonies are about 1 mm in diameter, beige, opaque, flat, circular with a smooth surface and an entire edge; cells are plump rods that are 1 to 2 µm long and about 0.5 µm wide. No anaerobic growth on NAB, TSA or TSA supplemented with 10 mM KNO3. Growth on rich medium is inhibited between 36 and 37 °C. A clear growth rate reduction was observed at temperatures between 30 and 35 °C. Growth at 15, 20 and 28 °C on TSA, but not at 4, 37, 40 or 45 °C after 7 days of incubation; growth at 15, 20, 28 and 37 °C (weak) on NAB, but not 4, 40 or 45 °C after 7 days of incubation. Growth in the presence of 0 and 1% NaCl, but not 2 % or more. Growth at pH 6 and 7, but not at pH 4.0, 5.0, 8.0 or 9.0.
The preferred carbon sources of strain Msb3T are simple C4 sugars. The strain can grow in minimal medium that contains glucose as well as malate and citrate as sole carbon sources. At an equimolar concentration growth on malate is more efficient than growth on glucose. Growth on MacConkey and Drigalski agar, but not on Pseudomonas cetrimide agar. Growth on Tween 20, 40, 60 and 80 agar base; hydrolysis of Tween 20, 40, 60 and 80 was apparent after 3 days of incubation only. No DNase activity or hydrolysis of starch or casein. Hemolysis on horse blood agar was negative after 2 days of incubation but positive after 5 days of incubation. Catalase and oxidase activity are present.
When examined using the API 20NE microtest system, the following characteristics were present in strain Msb3T: aesculin hydrolysis, assimilation of l-arabinose (weakly), d-glucose, d-mannose, d-mannitol, N-acetyl-glucosamine, potassium gluconate, adipic acid, malate, caprate and trisodium citrate but not nitrate reduction, indol production from l-tryptophane, beta-galactosidase (ONPG), fermentation of glucose, activity of arginine dihydrolase, urease and gelatin liquefaction, and assimilation of maltose or phenylacetic acid.
When examined using the API ZYM microtest system, the following characteristics were present in strain Msb3T: butyrate esterase (C4) (weak), caprylate esterase lipase (C8) (weak), alkaline and acid phosphatase, phosphoamidase and leucyl arylamidase, but not myristate lipase (C14), valine arylamidase, cystin arylamidase, trypsin, chymotrypsin, alpha-galactosidase, beta-galactosidase, beta-glucuronidase, alpha-glucosidase, beta-glucosidase, N-acetyl-beta-glucosaminidase, alpha-mannosidase or alpha-fucosidase.
Strain Msb3T is resistant to several commonly used antibiotics including ampicillin (50–100 µg ml−1), cefalexin (10–40 µg ml−1), and chloramphenicol (25 µg ml−1). It is, however, sensitive to standard inhibitory concentrations of carbenicillin, streptomycin as well as kanamycin.
The type strain is Msb3T (=LMG 31881T, DSM 111632T, CECT 30342T), which was isolated in December 2017 from leaf acumens of Dioscorea bulbifera. The whole-genome sequence of strain Msb3T has a size of 8.35 Mbp and consists of two chromosomes and three plasmids. The G+C content is 62.5 mol%. This whole genome sequence is publicly available under accession numbers LR699553-LR699557 (BioProject PRJEB33427). The 16S rRNA gene sequence of strain Msb3T is publicly available through the GenBank/EMBL/DDBJ accession number LR861108.
Supplementary Data
Funding information
The authors received no specific grant from any funding agency.
Acknowledgements
We thank Margo Cnockaert for technical assistance. The Vienna Biocenter Core Facility (VBCF) (University of Vienna) is acknowledged for providing essential services for the generation of the presented sequencing data. We are grateful for the hard work of our gardeners Andreas Schröfl and Thomas Joch.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: ANI, average nucleotide identity; CDS, coding sequence; COG, cluster of orthologous groups of proteins; dDDH, digital DNA–DNA hybridization; GI, genomic island; MALDI-TOF MS, matrix-assisted laser desorption/ionization-time of flight mass spectrometry; NAB, buffered nutrient agar; PAH, polycyclic aromatic hydrocarbon; PCB, polychlorinated biphenyl; TSA, trypticase soy agar; TYGS, Type (Strain) Genome Server.
A supplementary table is available with the online version of this article.
References
- 1.Sawana A, Adeolu M, Gupta RS. Molecular signatures and phylogenomic analysis of the genus Burkholderia: proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Front Genet. 2014;5:429. doi: 10.3389/fgene.2014.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim JH, Baek S-H, Lee S-T. Burkholderia sediminicola sp. nov., isolated from freshwater sediment. Int J Syst Evol Microbiol. 2008;58:565–569. doi: 10.1099/ijs.0.65502-0. [DOI] [PubMed] [Google Scholar]
- 3.Aizawa T, Bao Ve N, Vijarnsorn P, Nakajima M, Sunairi M. Burkholderia acidipaludis sp. nov., aluminium-tolerant bacteria isolated from Chinese water chestnut (Eleocharis dulcis) growing in highly acidic swamps in South-East Asia. Int J Syst Evol Microbiol. 2010;60:2036–2041. doi: 10.1099/ijs.0.018283-0. [DOI] [PubMed] [Google Scholar]
- 4.Aizawa T, Ve NB, Nakajima M, Sunairi M. Burkholderia heleia sp. nov., a nitrogen-fixing bacterium isolated from an aquatic plant, Eleocharis dulcis, that grows in highly acidic swamps in actual acid sulfate soil areas of Vietnam. Int J Syst Evol Microbiol. 2010;60:1152–1157. doi: 10.1099/ijs.0.015198-0. [DOI] [PubMed] [Google Scholar]
- 5.Rusch A, Islam S, Savalia P, Amend JP. Burkholderia insulsa sp. nov., a facultatively chemolithotrophic bacterium isolated from an arsenic-rich shallow marine hydrothermal system. Int J Syst Evol Microbiol. 2015;65:189–194. doi: 10.1099/ijs.0.064477-0. [DOI] [PubMed] [Google Scholar]
- 6.Herpell JB, Schindler F, Bejtović M, Fragner L, Diallo B, et al. The potato yam phyllosphere ectosymbiont Paraburkholderia sp. Msb3 is a potent growth promotor in tomato. Front Microbiol. 2020;11:581. doi: 10.3389/fmicb.2020.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deris ZZ, Van Rostenberghe H, Habsah H, Noraida R, Tan GC, et al. First isolation of Burkholderia tropica from a neonatal patient successfully treated with imipenem. Int J Infect Dis. 2010;14:e73–4. doi: 10.1016/j.ijid.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Moulin L, Munive A, Dreyfus B, Boivin-Masson C. Nodulation of legumes by members of the β-subclass of Proteobacteria . Nature. 2001;411:948–950. doi: 10.1038/35082070. [DOI] [PubMed] [Google Scholar]
- 9.Gao Z-H, Zhong S-F, Lu Z-E, Xiao S-Y, Qiu L-H. Paraburkholderia caseinilytica sp. nov., isolated from the pine and broad-leaf mixed forest soil. Int J Syst Evol Microbiol. 2018;68:1963–1968. doi: 10.1099/ijsem.0.002774. [DOI] [PubMed] [Google Scholar]
- 10.Gao Z-H, Ruan S-L, Huang Y-X, Lv Y-Y, Qiu L-H. Paraburkholderia phosphatilytica sp. nov., a phosphate-solubilizing bacterium isolated from forest soil. Int J Syst Evol Microbiol. 2019;69:196–202. doi: 10.1099/ijsem.0.003129. [DOI] [PubMed] [Google Scholar]
- 11.Otsuka Y, Muramatsu Y, Nakagawa Y, Matsuda M, Nakamura M, et al. Burkholderia oxyphila sp. nov., a bacterium isolated from acidic forest soil that catabolizes (+)-catechin and its putative aromatic derivatives. Int J Syst Evol Microbiol. 2011;61:249–254. doi: 10.1099/ijs.0.017368-0. [DOI] [PubMed] [Google Scholar]
- 12.Wilhelm RC, Murphy SJL, Feriancek NM, Karasz DC, DeRito CM, et al. Paraburkholderia madseniana sp. nov., a phenolic acid-degrading bacterium isolated from acidic forest soil. Int J Syst Evol Microbiol. 2020;70:2137–2146. doi: 10.1099/ijsem.0.004029. [DOI] [PubMed] [Google Scholar]
- 13.Yang H-C, Im W-T, Kim KK, An D-S, Lee S-T. Burkholderia terrae sp. nov., isolated from a forest soil. Int J Syst Evol Microbiol. 2006;56:453–457. doi: 10.1099/ijs.0.63968-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Gong G, Min Woo H, Kim Y, Um Y. Burkholderia jirisanensis sp. nov., isolated from forest soil. Int J Syst Evol Microbiol. 2016;66:1260–1267. doi: 10.1099/ijsem.0.000867. [DOI] [PubMed] [Google Scholar]
- 15.Chain PS, Denef VJ, Konstantinidis KT, Vergez LM, Agullo L, et al. Burkholderia xenovorans LB400 harbors a multi-replicon, 9.73-Mbp genome shaped for versatility. Proc Natl Acad Sci U S A. 2006;103:15280–15287. doi: 10.1073/pnas.0606924103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coenye T, Henry D, Speert DP, Vandamme P. Burkholderia phenoliruptrix sp. nov., to Accommodate the 2,4,5-Trichlorophenoxyacetic Acid and Halophenol-Degrading Strain AC1100. Syst Appl Microbiol. 2004;27:623–627. doi: 10.1078/0723202042369992. [DOI] [PubMed] [Google Scholar]
- 17.González PS, Ontañon OM, Armendariz AL, Talano MA, Paisio CE, et al. Brassica napus hairy roots and rhizobacteria for phenolic compounds removal. Environ Sci Pollut Res Int. 2013;20:1310–1317. doi: 10.1007/s11356-012-1173-9. [DOI] [PubMed] [Google Scholar]
- 18.Goris J, De Vos P, Caballero-Mellado J, Park J, Falsen E, et al. Classification of the biphenyl- and polychlorinated biphenyl-degrading strain LB400T and relatives as Burkholderia xenovorans sp. nov. Int J Syst Evol Microbiol. 2004;54:1677–1681. doi: 10.1099/ijs.0.63101-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y, Jeon CO. Paraburkholderia aromaticivorans sp. nov., an aromatic hydrocarbon-degrading bacterium, isolated from gasoline-contaminated soil. Int J Syst Evol Microbiol. 2018;68:1251–1257. doi: 10.1099/ijsem.0.002661. [DOI] [PubMed] [Google Scholar]
- 20.Esmaeel Q, Miotto L, Rondeau M, Leclère V, Clément C, et al. Paraburkholderia phytofirmans PsJN-Plants Interaction: from perception to the induced mechanisms. Front Microbiol. 2018;9:2093. doi: 10.3389/fmicb.2018.02093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitter B, Petric A, Shin MW, Chain PS, Hauberg-Lotte L, et al. Comparative genome analysis of Burkholderia phytofirmans PsJN reveals a wide spectrum of endophytic lifestyles based on interaction strategies with host plants. Front Plant Sci. 2013;4:120. doi: 10.3389/fpls.2013.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman M, Sabir AA, Mukta JA, Khan MMA, Mohi-Ud-Din M, et al. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci Rep. 2018;8:2504. doi: 10.1038/s41598-018-20235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sessitsch A, Coenye T, Sturz AV, Vandamme P, Barka EA, et al. Burkholderia phytofirmans sp. nov., a novel plant-associated bacterium with plant-beneficial properties. Int J Syst Evol Microbiol. 2005;55:1187–1192. doi: 10.1099/ijs.0.63149-0. [DOI] [PubMed] [Google Scholar]
- 24.Vandamme P, Opelt K, Knochel N, Berg C, Schonmann S, et al. Burkholderia bryophila sp. nov. and Burkholderia megapolitana sp. nov., moss-associated species with antifungal and plant-growth-promoting properties. Int J Syst Evol Microbiol. 2007;57:2228–2235. doi: 10.1099/ijs.0.65142-0. [DOI] [PubMed] [Google Scholar]
- 25.Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 26.Aizawa T, Vijarnsorn P, Nakajima M, Sunairi M. Burkholderia bannensis sp. nov., an acid-neutralizing bacterium isolated from torpedo grass (Panicum repens) growing in highly acidic swamps. 2011;61:1645–1650. doi: 10.1099/ijs.0.026278-0. [DOI] [PubMed] [Google Scholar]
- 27.Vacca DJ, Bleam WF, Hickey WJ. Isolation of soil bacteria adapted to degrade humic acid-sorbed phenanthrene. Appl Environ Microbiol. 2005;71:3797–3805. doi: 10.1128/AEM.71.7.3797-3805.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier-Kolthoff JP, Goker M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun. 2019;10:2182. doi: 10.1038/s41467-019-10210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meier-Kolthoff JP, Auch AF, Klenk HP, Goker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. Bmc Bioinformatics. 2013;14:60. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon SH, Ha SM, Lim J, Kwon S, Chun J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie van Leeuwenhoek. 2017;110:1281–1286. doi: 10.1007/s10482-017-0844-4. [DOI] [PubMed] [Google Scholar]
- 31.Dupont CL, Rusch DB, Yooseph S, Lombardo MJ, Richter RA, et al. Genomic insights to SAR86, an abundant and uncultivated marine bacterial lineage. Isme J, Article. 2012;6:1186–1199. doi: 10.1038/ismej.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ankenbrand MJ, Keller A. bcgTree: automatized phylogenetic tree building from bacterial core genomes. Genome. 2016;59:783–791. doi: 10.1139/gen-2015-0175. [DOI] [PubMed] [Google Scholar]
- 33.Letunic I, Bork P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019;47:W256–W259. doi: 10.1093/nar/gkz239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eren AM, Kiefl E, Shaiber A, Veseli I, Miller SE, et al. Community-led, integrated, reproducible multi-omics with anvi’o. Nat Microbiol. 2021;6:3–6. doi: 10.1038/s41564-020-00834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galperin MY, Wolf YI, Makarova KS, Vera Alvarez R, Landsman D, et al. COG database update: Focus on microbial diversity, model organisms, and widespread pathogens. Nucleic Acids Research. 2020;49:D274–D281. doi: 10.1093/nar/gkaa1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagy I, Schoofs G, Compernolle F, Proost P, Vanderleyden J. Degradation of the thiocarbamate herbicide EPTC (S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J Bacteriol. 1995;177:676–687. doi: 10.1128/jb.177.3.676-687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickey WJ, Chen S, Zhao J. The phn Island: A new genomic island encoding catabolism of polynuclear aromatic hydrocarbons. Front Microbiol. 2012;3:125. doi: 10.3389/fmicb.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Research. 2015;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricci JN, Coleman ML, Welander PV, Sessions AL, Summons RE, et al. Diverse capacity for 2-methylhopanoid production correlates with a specific ecological niche. <span data-cid="1627561378" id="f99dd15f-0fd6-490b-ac7c-66f43ba8938a" title="UPPERCASE Added by Lavanya (PREEDITOR) - 07/29/2021 6. 8:675–684. doi: 10.1038/ismej.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malott RJ, Baldwin A, Mahenthiralingam E, Sokol PA. Characterization of the cciIR quorum-sensing system in Burkholderia cenocepacia . Infect Immun. 2005;73:4982–4992. doi: 10.1128/IAI.73.8.4982-4992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramoni S, Sokol PA. Quorum sensing systems influence Burkholderia cenocepacia virulence. Future Microbiol. 2012;7:1373–1387. doi: 10.2217/fmb.12.118. [DOI] [PubMed] [Google Scholar]
- 42.Xie M, Chung CY-L, Li M-W, Wong F-L, Wang X, et al. A reference-grade wild soybean genome. Nat Commun. 2019;10:1216. doi: 10.1038/s41467-019-09142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacFaddin JF. Biochemical Tests for Identification of Medical Bacteria. 3rd ed. Baltimore (Md: Williams and Wilkins; 2000. [Google Scholar]
- 44.Dumolin C, Aerts M, Verheyde B, Schellaert S, Vandamme T, et al. Introducing SPeDE: high-throughput dereplication and accurate determination of microbial diversity from matrix-assisted laser desorption-ionization time of flight mass spectrometry data. mSystems. 2019;4:e00437-00419. doi: 10.1128/mSystems.00437-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akimowicz M, Bucka-Kolendo J. MALDI-TOF MS - application in food microbiology. Acta Biochim Pol. 2020;67:327–332. doi: 10.18388/abp.2020_5380. [DOI] [PubMed] [Google Scholar]
- 46.Doern CD, Butler-Wu SM. Emerging and future applications of matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry in the clinical microbiology laboratory: A report of the association for molecular pathology. J Mol Diagn. 2016;18:789–802. doi: 10.1016/j.jmoldx.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Rahi P, Prakash O, Shouche YS. Matrix-assisted laser desorption/ionization time-of-flight mass-spectrometry (MALDI-TOF MS) based Microbial identifications: Challenges and scopes for microbial ecologists. Front Microbiol. 2016;7:1359. doi: 10.3389/fmicb.2016.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tindall BJ, Rosselló-Móra R, Busse H-J, Ludwig W, Kämpfer P. Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol. 2010;60:249–266. doi: 10.1099/ijs.0.016949-0. [DOI] [PubMed] [Google Scholar]
- 49.Strohalm M, Kavan D, Novák P, Volný M, Havlícek V. Mmass 3: A cross-platform software environment for precise analysis of mass spectrometric data. Anal Chem. 2010;82:4648–4651. doi: 10.1021/ac100818g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.