Abstract
Study Objectives
COVID-19 lockdowns drastically affected sleep, physical activity, and wellbeing. We studied how these behaviors evolved during reopening the possible contributions of continued working from home and smartphone usage.
Methods
Participants (N = 198) were studied through the lockdown and subsequent reopening period, using a wearable sleep/activity tracker, smartphone-delivered ecological momentary assessment (EMA), and passive smartphone usage tracking. Work/study location was obtained through daily EMA ascertainment.
Results
Upon reopening, earlier, shorter sleep and increased physical activity were observed, alongside increased self-rated stress and poorer evening mood ratings. These reopening changes were affected by post-lockdown work arrangements and patterns of smartphone usage. Individuals who returned to work or school in-person tended toward larger shifts to earlier sleep and wake timings. Returning to in-person work/school also correlated with more physical activity. Contrary to expectation, there was no decrease in objectively measured smartphone usage after reopening. A cluster analysis showed that persons with relatively heavier smartphone use prior to bedtime had later sleep timings and lower physical activity.
Conclusions
These observations indicate that the reopening after lockdown was accompanied by earlier sleep timing, increased physical activity, and altered mental wellbeing. Moreover, these changes were affected by work/study arrangements and smartphone usage patterns.
Keywords: COVID-19, lockdown, sleep, physical activity, wellbeing, wearable, smartphone
Statement of Significance.
The COVID-19 pandemic disrupted daily lives worldwide, impacting sleep, physical activity, and mental wellbeing. Although lockdowns were imposed suddenly, the process of reopening has often been more gradual with partial mobility restrictions still in place. By longitudinally tracking sleep, physical activity, and wellbeing through lockdown and the extended period of reopening, we document how these are affected by persistence of working from home and pre-bedtime smartphone usage. As hybrid work arrangements and expanded use of electronic communication are likely to persist through the protracted pandemic and its aftermath, our findings could inform strategies to adapt to new societal norms.
Introduction
The COVID-19 pandemic that struck the world in February 2020 has drastically altered the lives of billions of people across the globe. Worldwide, lockdowns, comprising social and mobility restrictions and closures of workplaces and schools, have been instituted to stem the spread of the SARS-Cov2 virus. The sudden imposition of restricted mobility and social interaction strongly disrupted people’s daily routines and wellbeing. In the early stages of the pandemic, marked reductions in physical activity as a result of home confinement were documented, with daily step counts dropping from the recommended 10 000/day to nearly half [1–3]. At the same time, reports of stress and anxiety about the pandemic situation, and loneliness due to social isolation increased [4–6]. On the other hand, working from home has resulted in less rigidly stipulated work hours and less time spent on routine activities, such as commuting [7]. This increased flexibility in our daily schedules has contributed to changes in our sleep habits, resulting in later sleep timing and longer sleep duration [8–12], but also poorer sleep quality and higher reports of insomnia symptoms [13, 14]. These changes have been robustly measured across a wide range of countries [15–18]. Furthermore, with face-to-face communication severely limited, people have turned to digital technology to stay connected for social interaction, work/education, and entertainment [19–22]. Many of these acute changes were associated with a negative impact on health and mental wellbeing [5, 15, 23–26].
In contrast to the abruptness of lockdowns, the process of reopening has generally been more gradual and phased. Often some social distancing measures continued to be kept in place for an extended period of time after the lockdown was lifted [27, 28]. At the time of writing, one and a half years into the pandemic, it has become increasingly clear that there will be no quick resolution to the pandemic. The need for intermittent restriction measures (e.g. hybrid in-person/remote work forms) may persist for an extended duration [29, 30], and many countries have experienced multiple cycles of lockdown and reopening. In this light, it is important to collect longitudinal data to evaluate health behavior and wellbeing outcomes over time, identify potentially mitigating and exacerbating factors, and formulate effective intervention strategies.
In this study, we objectively tracked lifestyle behaviors (physical activity, sleep, and phone use), and collected wellbeing data from a sample of students and working adults (N = 198) through the initial period of lockdown (April–May 2020) and subsequent reopening in Singapore (until September 2020). A nationwide lockdown (termed “Circuit Breaker”) was instituted from April 7th to June 1st, 2020. Lockdown measures consisted of closure of schools, nonessential workplaces, and public spaces, a ban on social gatherings and home visitors, and home confinement except for buying essential goods and services and for exercising [31]. In keeping with the global data, sharp reductions in physical activity, shifts to later and longer sleep, and decreases in wellbeing due to lockdown measures have been observed [11, 32–35].
A cautious and phased reopening strategy was adopted at the end of the lockdown period. From June 2nd onward, schools were reopened, and households could receive up to two visitors per day [36]. On June 19th, measures were further eased, allowing more in-person classes and work arrangements, reopening of physical retail, dining, and sports facilities, and an increased maximum group size of five persons (applying to public spaces and home visitors) [37]. Several safety measures (e.g. mask wearing, physical distance, adherence to track-and-trace measures in public spaces), were kept in place until the end of the year (December 2020), when further easing of these was announced [38].
Our data collection overlapped with the transition from lockdown to phased reopening. Daily records of sleep, physical activity, phone use, and wellbeing were collected through a combination of wearable, smartphone tracking, and ecological momentary assessment (EMA) technology. This allowed us to longitudinally monitor detailed changes over time, in particular changes related to the reopening. The inclusion of wearable and smartphone tracking technology provided objective measurements (circumventing potential recall and reporting issues common in subjective—often retrospective—survey studies), at a minimal burden to participants.
As the study was initiated in the early pandemic stages (prior to the institution of lockdown), no prior studies were available to base a priori hypotheses on. However, a rapid accumulation of knowledge has provided some robust data on the effects of lockdowns on lifestyle patterns (e.g. reduced physical activity, shifted sleep patterns, increased e-device usage). Based on these insights, it could be expected that lifting of the lockdown would lead to a (partial) reversal of these effects. In our analysis, we examined the contribution of continued work-from-home (vs. return to in-person work), and night-time mobile phone usage to behavior as these factors have been found to modulate sleep and wellbeing during lockdown periods [39–41].
Methods
Participants and procedure
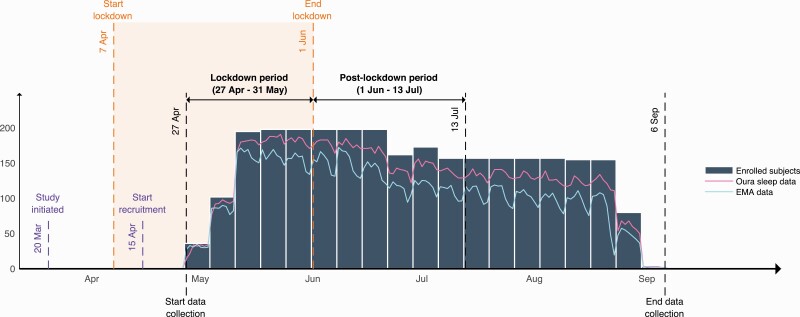
A total of 200 young adults, working or studying at the National University of Singapore or working at the National University Hospital of Singapore, were recruited via online platforms to participate in an observational remote-tracking study on sleep and wellbeing during the COVID-19 pandemic. Recruitment started during the lockdown (start lockdown: April 7th; start recruitment: April 15th, 2020; see Figure 1), and enrollment was carried out on a rolling basis over a 4-week time span. Participants were briefed on study procedures remotely via a short instructional video and signed electronic informed consent, after which they were sent a sleep and activity tracking device (Oura ring) via courier. For each participant, data collection started on the Monday after they had received their sleep tracking device, resulting in four recruitment batches with staggered starting dates (see Figure 1; Batch 1: N = 37, start date: 27th April; Batch 2: N = 67, start date: 4th May; Batch 3: N = 93, start date: 11th May; Batch 4: N = 3, start date: 18th May). Participants were initially enrolled for an 8-week monitoring period and were later given the option to extend their participation with another 8 weeks (16 weeks in total). Forty-one participants exited after the initial 8 weeks, and 157 participants re-consented for 16 weeks. Two participants withdrew from the study before completing the initial 8-week period, leaving 198 (staff: N = 78, students: N = 120, Mage = 26.15 ± 5.83 years, 61 male) in the final sample.
Figure 1.
Timeline of lockdown, reopening and study events, illustrating the number of participants enrolled and data completion over the study period.
During the monitoring period, participants were instructed to wear the Oura ring at all times for the collection of sleep and physical activity data. Twice daily, they were prompted to complete short questionnaires and cognitive assessment games through an EMA application on their phones (daytime window [8 am–5 pm]: questionnaires and cognitive games; evening window [8 pm–12 am]: questionnaires only). Phone use records were passively provided through the phone usage tracking app (TapCounter, Quantactions GmbH [42]). In addition, every 4 weeks from enrolment, participants were invited to complete a set of online surveys (including questions about their work hours, daily routines, causes of stress, and comfort of the wearable trackers over the previous 4 weeks).
To motivate compliance, participants could earn $10 per week for logging the minimum of 4 days of data on all study applications (i.e. four nights of sleep data via Oura + 4 days of EMA data + 4 days of phone use data for each week). An additional $20 bonus was provided upon completion of the study and return of the Oura ring. Feedback on completion rates and rewards accrued was provided after each week. Ethical approval for all study procedures was obtained from the National University of Singapore Institutional Review Board (NUS-IRB Ref Code: N-20-039).
Sleep metrics
Wearable-based sleep measures were extracted daily from the commercial Oura ring (Oura, Health Oy, Oulu, Finland). This device tracks users’ heart rate, temperature changes, and movement through photoplethysmography sensors, temperature sensors, and an accelerometer.
Sleep outcome variables provided by Oura included bedtime, wake times, time-in-bed (TIB), and total sleep time (TST). Sleep efficiency was calculated as 100 × TST/TIB, and mid-sleep times (MST) were calculated as the midpoint between bedtimes and wake times on the same night. Social jetlag was calculated as the difference between weekday and weekend MST (MSTWE − MSTWD). The Oura ring additionally provides sleep stage information (light sleep, deep sleep, rapid eye movement), however, this information was not used in our analyses. Nap information collection was not possible with the firmware version available at the time of testing. Instead, self-reported nap data were collected through EMA.
Validation studies have reported generally good accuracy of sleep-wake detection for the Oura ring, with small absolute error for TST estimation compared to polysomnography (PSG; 87.8% of nights within 30-minutes error) [43], and ambulatory electroencephalography (7.39% mean absolute percentage error) [44]. Modest systematic error of Oura-derived TST have been found in adult populations (approximately 15 min overestimation) compared to PSG [45], and actigraphy [46], while larger systematic underestimation was found in adolescents [47].
Daily self-reports of sleep timings and ratings of sleep quality on a 5-point Likert scale (0 = Very poor to 4 = Very good), were also collected through EMA. A third measure of sleep timing was derived from an application that recorded participant’s screen interactions and thus mobile device usage (tappigraphy) throughout the day. These were used to corroborate sleep and wake timings obtained using the Oura ring, reported elsewhere [48].
Physical activity
Step count as extracted from the Oura ring was used as an indicator of daily physical activity. Based on the daily non-wear time provided by Oura, we excluded step counts on days during which non-wear time exceeded 3 h. Participants who had only worn the device at night and not during the day were excluded from step count analysis (lockdown N = 2, post-lockdown N = 7).
Mobile device usage
Smartphone usage was measured using a smartphone application that passively tracked touchscreen interactions and screen on/off events, as well as the name of the active application (TapCounter by QuantActions GmbH, Lausanne, Switzerland, www.quantactions.com) [42]. Data from one participant who used an auto tap generation application was removed from analyses of mobile device usage.
Wellbeing and performance
Participants rated their levels of stress and mood twice daily (once in the morning and once in the evening), and loneliness thrice a week (once in the evening every Monday, Thursday, and Saturday) in response to a short questionnaire administered on the EMA phone application. Responses were collected on a rating scale of 0 to 100 (0 = Not at all, 100 = Very much for stress and loneliness scales, 0 = Negative, and 100 = Positive for mood scale). In conjunction with the morning questionnaire, participants completed a short set of three cognitive assessments daily: 3-min psychomotor vigilance test, dot memory test, and symbol search test (adapted from Ref. [49]). Data from these tests are not presented here.
Work location
Participants were asked to report the extent to which they returned to their workplace/school during the lockdown in the survey administered at the end of the study (“How frequently did you go to the office/hospital/school/other workplace during the lockdown?”). To account for varying work arrangements post-lockdown, a daily question enquiring participants’ work location on that day was included in the EMA upon reopening (“From what location did you work/attend lessons today?”). Participants could select from the four options: “Home,” “Office/hospital/university,” “Others,” or “Did not have to work/attend lessons.” In the present analyses, the two categories: “Office/hospital/university” and “Others” were collapsed into one “In-person” work category.
Analyses
To examine changes in lifestyle and wellbeing associated with the reopening, we focused on data collected in two periods during study protocol: (1) during the lockdown (April 27th–May 31st, 2020), and (2) during the first 6 weeks post-lockdown (June 1st–July 13th, 2020), as all participants contributed at least 2 weeks of data on all measures within both stated periods. Given the staggered starting dates for data collection, however, the number of days each participant contributed varied across lockdown and post-lockdown periods (lockdown: mean number of days (SD) = 25.98 (5.80); post-lockdown: mean number of days (SD) = 36.84 (6.93); for a detailed breakdown see Supplementary Table S1). Individual average scores were calculated for sleep metrics, physical activity, phone use, and wellbeing ratings, separately for the lockdown (April–May) and post-lockdown period (June–July). Resulting change scores were compared using two-tailed paired sample t-tests and nonparametric Wilcoxon signed-rank tests in cases where the assumption of normality was violated. Although reopening effects could be expected show a reversed pattern as the effects of lockdown instatement, the precise occurrence and magnitude of reopening effects were unknown prior to analysis (as the effects of reopening in the larger societal context were unknown). To correct for multiple testing, while still allowing discovery of novel results among a wide array of wellbeing and lifestyle variables, the Benjamini–Hochberg procedure [50] was applied with false discovery rate of 10% (FDR = .1). Effect sizes were calculated and interpreted following common rules of thumb (Cohen’s d: small: 0.2, medium: 0.5, large: 0.8; r [for nonparametric tests]: small: 0.1, medium: 0.3, large: 0.5) [51].
Effects of post-lockdown work location
Some studies have found that work arrangements during lockdown (in-person vs. work-from-home) affected sleep and wellbeing during lockdown [40, 52]. We set out to test how changes in lifestyle and well-being after reopening were moderated by participants’ post-lockdown work arrangements. Daily work location reports, provided via EMA, were used to calculate the proportion of days worked in-person (over the total number of working days). To examine the effects of returning to in-person work arrangements, we performed regression analyses for the sleep, physical activity, and wellbeing variables as dependent variables, and the proportion of on-campus/in-office work versus work-from-home days as a predictor (controlled for demographics: age, gender, student/staff; see Supplementary Tables S2 and S3). All tests were two-tailed, and results were corrected for multiple testing using the Benjamini–Hochberg method with FDR = .1.
Identification of bedtime phone usage patterns
Phone usage at bedtime is known to affect sleep before and during the pandemic [25, 41, 53, 54]. To assess bedtime phone usage patterns, individuals’ average phone use time in the period surrounding their bedtime (in 10 min time bins starting 3 h before bedtime till 1 h after) were calculated, across lockdown and post-lockdown periods separately. Resulting bedtime phone use profiles were entered into a clustering analysis using the k-means++ algorithm (Matlab version 2017b, Mathworks, Natick, MA) with the squared Euclidean distance metric for center initialization of clusters. Based on the elbow plots, obtained from assessing within-cluster sums of squared distance of various cluster numbers (k = 2–10), an optimal number of clusters was identified. Identification of phone-use clusters was data-driven and no a priori predictions about the optimal number or configuration of the cluster could be made. Subjects in the resulting clusters were then compared using regression analyses with the sleep, physical activity, and wellbeing variables as dependent variables, and cluster group as a predictor (controlling for demographics: age, gender, student/staff, and work arrangements; see Supplementary Tables S4–S7). Results were corrected for multiple comparisons using the Benjamini–Hochberg method with FDR = .1.
Results
Effects of reopening on lifestyle and well-being
Upon reopening, physical activity increased from an average of 5808 daily steps during lockdown to 7007 daily steps post-lockdown (mean Δ: +1199.1 steps/day; Z = −8.10; p < .001, r = −0.59; see Supplementary Table S2 for details). Average bedtime and wake time advanced from lockdown to post-lockdown by 14.1 min and 27.1 min respectively (bedtime: t = −4.01, p < .001, d = 0.29; wake time: t = 5.99, p < .001, d = 0.43). TIB and TST decreased from lockdown to post-lockdown (TIBΔ: −14.4 min, t = 6.83, p < .001, d = 0.49; TSTΔ: −11.4 min, t = 6.30, p < .001, d = 0.45), resulting from larger advances in wake times than in bedtimes. The difference between weekend and weekday mid sleep timing (social jetlag) increased with 7.9 min (Z = −3.28, p = .001, r = −0.23). Sleep quality ratings increased slightly but significantly from 2.56 points to 2.64 points (on a scale of 0 to 4; t = 4.34, p < .001, d = 0.31).
Concurrently, self-reported stress increased after reopening by 4.13 points in daytime ratings (Z = −5.41, p < .001, r = −0.38), and by 3.64 points in evening ratings (Z = −4.47, p < .001, r = −0.32). While these effect sizes were intermediate to large, the actual change scores represent only modest changes (approximately four points on a 100-point scale). Average mood score was significantly worsened at reopening only for evening ratings (Z = −2.65, p = .008, r = −0.19). No significant changes in loneliness were observed in self-reported loneliness ratings (p = .22).
Daily total smartphone usage (total screen unlock time over 24-hours) was not significantly different between the lockdown and reopening periods (approximately 5.7 h/day), while smartphone use at bedtime was slightly increased from 27.51 min (in the hour before bedtime) during lockdown, to 28.32 min post-lockdown (Z = −2.10, p = .036, r = −0.15). This increase is rather minimal and should not be taken as a substantial change. Importantly, however, there was no indication that smartphone use decreased after reopening (as was hypothesized following the reported increases during lockdown [19-22]).
The effect of work arrangements during reopening
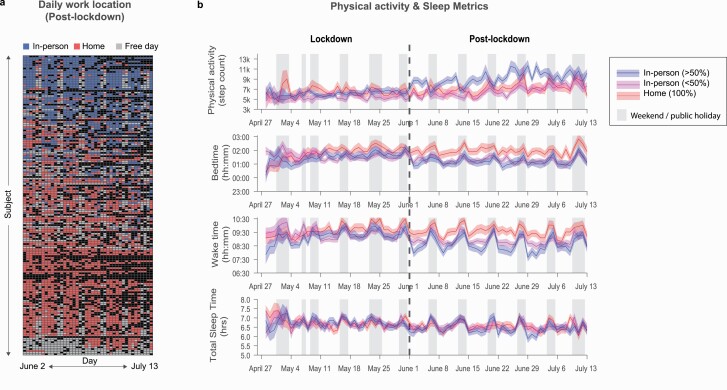
Daily post-lockdown work location reports, gathered through EMA, showed a variety of work arrangements over the first six weeks after reopening (see Figure 2A). Most participants showed a mix of working-from home and in-person work/study to various degrees (N = 113). However, about a third continued to work-from-home fully (N = 61). Of the participants who returned to their workplaces, N = 58 reported to work in-person 50% of the time or less, while the other N = 55 worked in-person on a majority of working days (>50%; see Figure 2B). An additional 24 participants reported no working days during the post-lockdown period and were excluded from further analyses.
Figure 2.
The effect of timeseries of lifestyle variables over the lockdown and post-lockdown periods, stratified by the proportion of days worked-from-home. (A) Daily work location reports collected through EMA. (B) Time series of physical activity and sleep over the lockdown and post-lockdown period. Data split by work location 100% work-from-home, <50% work in-office, >50% work in-office for visualization purposes.
Regression analyses revealed that the proportion of days worked in-person was associated with significantly earlier sleep timing (bedtime B = −0.66, p = .011, [−1.18, −0.15]; wake time B = −0.90, p = .003, [−1.49, −0.32]; see Figure 2B and Supplementary Table S3a), but not with TST or social jetlag (ps > .50). This indicates that individuals who fully went back to in-person work slept 39.6 min earlier and woke 54 min earlier than those who worked-from-home 100% of the time. More in-person work was also associated with increased daily step count, such that individuals who worked in-person every day logged almost 2000 steps per day more than those who worked-from-home fully (B = 1911.81, p = .009, [478.34, 3345.29]). While individuals reporting more in-person work also reported slightly worse morning and evening mood (morning B = −5.76, p = .043, [−11.32, −0.19]; evening B = −5.467, p = .044, [−10.79, −0.14]), these associations were small (the estimated difference between individuals working in-person 100% and those working from home fully was just over five points on a 100-point scale). No associations with other wellbeing variables were found (see Supplementary Table S3b for details). As the proportion of days worked in-person was not normally distributed (i.e. about one-third of participants did not return to office/campus), we performed a control analysis using 100% at-home, less than 50% in-person, and more than 50% in-person participants as separate groups. These analyses produced highly similar findings (see Supplementary Tables S4a and b).
The effect of phone use on sleep, physical activity, and wellbeing
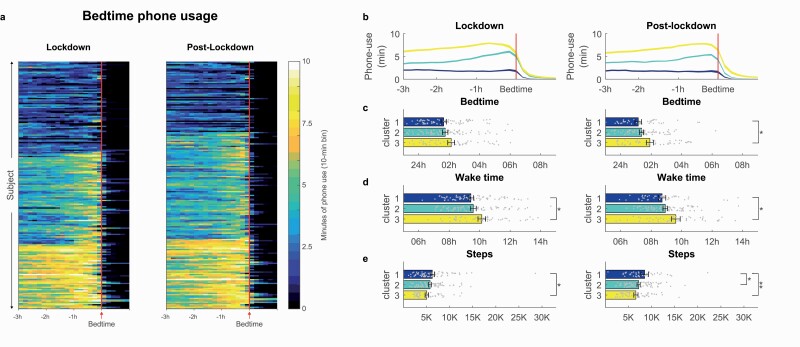
Cluster analyses of individuals’ average phone usage around bedtime (3 h before bedtime to 1 h after bedtime), revealed three distinct phone usage profiles (see Figure 3A). Cluster 1 (Nlockdown = 73, Npost-lockdown = 56) was characterized by low amounts of phone usage throughout the period before bedtime (see Figure 3B). Cluster 2 (Nlockdown = 72, Npost-lockdown = 85) showed moderate levels of phone usage at the start of the night, which ramped up in the hour before bedtime. For Cluster 3 individuals (Nlockdown = 52, Npost-lockdown = 56), high levels of phone usage were observed across the 3 h before bedtime. Examining specific types of apps used, Clusters 2 and 3 primarily showed an increase of social media, and browser apps toward bedtime, while Cluster 1 showed consistent low usage across all apps (see Supplementary Figure S1).
Figure 3.
Bedtime phone usage profiles during lockdown and post-lockdown, with (A) Heat plot indicating peri-bedtime phone usage, identifying three distinct clusters of phone usage profiles, (B) cluster average timeseries of bedtime phone usage, (C) bedtime, (D) wake time, and (E) physical activity. *p < .05, **p < .01. Error bars represent standard error of the mean.
Regression analyses, controlling for demographics and work arrangements, indicated that these different bedtime phone use clusters were associated with differences in sleep timing and physical activity (see Figure 3C–E). Individuals with low bedtime phone usage (Cluster 1) had earlier wake times during lockdown (09:25 am ± 01:30 hh:mm) compared to high bedtime phone users (Cluster 3: 10:08 am ± 01:49 hh:mm, B = 0.69, p = .015, 95% CI = [0.14 to 1.24]; see Supplementary Tables S5a and S7 for details). Post-lockdown, these differences between clusters were maintained for wake time (Cluster 1: 08:43 ± 01:36 hh:mm, Cluster 3: 09:37 ± 02:00 hh:mm, B = 0.80, p = .01, 95% CI = [0.19 to 1.41]; see Supplementary Tables S6a) as well as bedtime (Cluster 1: 01:09 am ± 01:27 hh:mm, Cluster 3: 01:55 am ± 01:37 hh:mm, B = 0.69, p = .012, 95% CI = [0.15 to 1.22]).
Individuals with low bedtime phone usage profiles logged more physical activity, both during lockdown (Cluster 1: 6253 ± 4029 steps, Cluster 3: 5040 ± 2544 steps, B = −1259.46, p = .033, 95% CI = [−2415.95 to −102.97]), and post-lockdown (Cluster 1: 8438 ± 5221, Cluster 2: 7111 ± 2971, B = −1581.97, p = .020, 95% CI = [−2913.95, −250.0]; Cluster 3: 6552 ± 3087, B = −2016.38, p = .009, 95% CI = [−3526.89 to −505.86]).
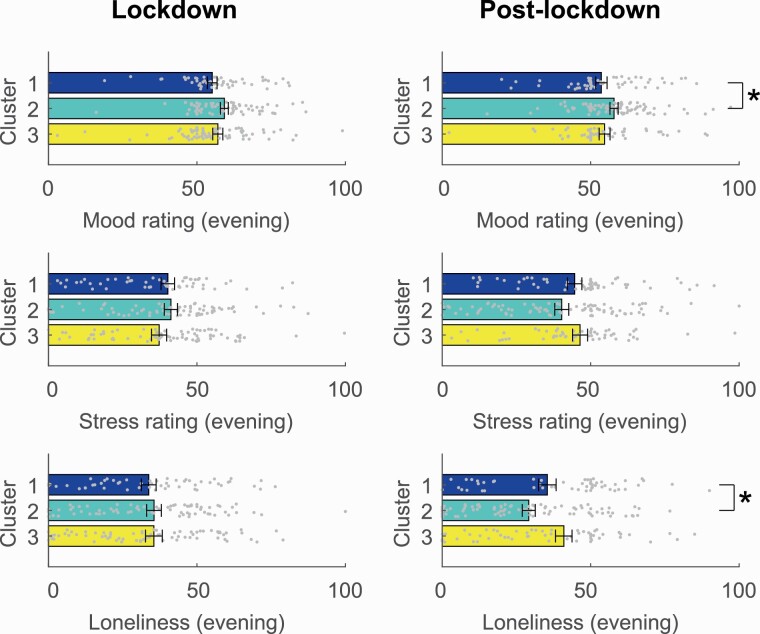
For mental wellbeing, however, associations were less strong (see Figure 4; Supplementary Tables S5b and S6b). Post-lockdown, the cluster with intermediate phone usage, phone use time ramping up closer to bedtime (Cluster 2) was associated with better mood in the evening (Cluster 1: mean = 53.41 ± 14.06; Cluster 2: mean = 58.69 ± 12.53, p = .025, [0.72 to 10.64]), as well as less loneliness (Cluster 1: mean = 36.93 ± 21.47; Cluster 2: mean = 28.41 ± 20.17, p = .025, [−16.59 to −1.13]). A similar pattern for evening mood during lockdown did not survive FDR correction for multiple comparisons. These effects suggest that individuals with moderate evening phone usage might have slightly better wellbeing, however, again these effects only represent a few points (5–8 points) on a 100-point scale.
Figure 4.
Wellbeing variables during lockdown and post-lockdown showing differences between phone use clusters. *p < .05. Error bars represent standard error of the mean.
Is the observed shift in sleep timings desirable?
Previous studies have speculated that shifts to later sleep timings found during the lockdown compared to a pre-lockdown baseline may be the result of increased flexibility of work schedules arising from remote working arrangements, thereby enabling individuals to follow their own preferred sleep schedule.[39] To probe this, participants were invited to report their preferred sleep timings in response to the question in our fourth-week survey: If you could choose your sleep schedule freely, what would be your… ideal bedtime/wake time/sleep duration?
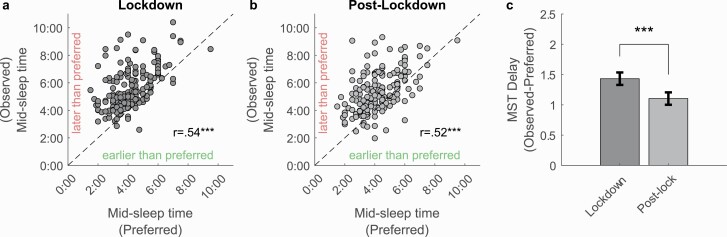
Ideal sleep timing, represented by mid-sleep times were calculated from responses as the midpoint between ideal bedtime and waketime (ideal mid-sleep time = ideal bedtime + (ideal wake time – ideal bedtime)/2). These were moderately correlated with the corresponding average actual observed midsleep timings across the lockdown period (r = 0.54, p < .001) as well as reopening period (r = 0.52, p < .001; Figure 5, A and B), suggesting that this measure was effective in capturing individual variations in preferred sleep timing. However, objective observed sleep timings tended to be delayed relative to participants self-reported ideal sleep timings, both during the lockdown (t(175) = 13.88, p < .001) as well as after reopening (t(175) = 10.75, p < .001). While this delay was reduced during reopening (t(175) = 5.52, p < .001), it remained significant, suggesting the persistence of other factors apart from lockdown-related changes such as work-from-home arrangements that delay actual sleep timings relative to preferred times. Furthermore, ideal mid-sleep time was inversely correlated with age (r = −0.27, 95% CI = [−0.40, to −0.12], p < .001), and was later for students (mean [SD] = 04:33 am [01:24 hh:mm]), compared to staff (mean [SD] = 03:45 am [01:01 hh:mm], t(175) = 4.16, p < .001, Cohen’s d = 0.64). Ideal mid-sleep time was unrelated to gender (t(175) = −1.065, p = .29, Cohen’s d = −0.18), or phone-use cluster (lockdown: F(3, 172) = 0.94, p = .43; post-lockdown: (F(3,172) = 1.09, p = .36).
Figure 5.
Comparison of objectively observed sleep timing versus self-reported preferred sleep timing. (A) Scatter plots of observed mid-sleep times against preferred sleep time in lockdown and (B) post-lockdown (dashed line indicates equality). (C) Discrepancy between observed and preferred mid-sleep time. ***p < .001. Error bars represent standard error of the mean.
Discussion
The current findings indicate that increased physical activity and earlier and shorter sleep were observed upon reopening after lockdown. These effects seem to reflect an inverse pattern of changes in lifestyle observed at the onset of lockdowns, as reported in a rapidly growing literature [1, 2, 8, 9, 11, 23, 40, 55, 56]. Interestingly, smartphone usage was not significantly reduced upon reopening as was predicted, and a slight increase in experienced stress and worsening of evening mood were observed. Shifts in physical activity, sleep, and wellbeing across the sample were associated with the degree to which individuals returned to in-person work after lockdown, as well as patterns of bedtime phone use.
Upon reopening, the majority of participants reported varying mixtures of in-person work and working-from-home, while a third of the sample continued to fully work-from-home. This heterogeneity in work arrangement is likely reflective of the phased reopening strategy that was adopted by the Singapore government (mandating continued remote work where possible in the first weeks after reopening) [57]. We found a clear relationship between the proportion of days worked from home versus in-person and lifestyle factors at post-lockdown. Sleep timing and daily step count returned towards more normal values for those individuals who reverted to in-person work most frequently. In contrast, participants who maintained working from home showed little change in activity and sleep timing after reopening.
Pandemic-related lockdowns across the world have revealed how much our going to work influences habitual physical activity and sleep. A recent population survey demonstrated that commute and work-related activities strongly contribute to total daily physical activity in Singaporean adults (44.5% and 30.0% respectively, vs. 25.5% for leisure-time physical activity) [58]. Such commute and work-related physical activity may have beneficial long-term health effects (e.g. reduced obesity risk) [59, 60]. Our data also concur with reports that working from home (vs. in-person work) is associated with later sleep timing [40, 52]. As daily schedules are less strongly dictated by office hours when working from home, time for other activities such as sleep could be more flexibly allocated [39]. This may be beneficial for health as the observed sleep patterns under less constrained schedules might reflect the individual’s biological tendencies [39]. However late sleep schedules, in general, are associated with negative health outcomes, and may reflect misalignment with natural night-day rhythms, due to reduced exposure to social and natural time cues [10, 40, 61]. It is worth noting that in our data nearly all participants indicated that under ideal circumstances they would prefer to sleep earlier than they typically did.
Given the observed effects of working from home on physical activity and sleep, it will be important to monitor the long-term progression of these behaviors, and to devise recommendations and programs to counteract negative outcomes of these sustained behavioral patterns. Globally, the pandemic forced hundreds of millions of people to work-from-home. As the situation unfolds, many countries have experienced extended lockdowns and/or multiple cycles of lockdown and reopening [27, 28, 62]. Technological viability and economic considerations have led businesses to further embrace remote work arrangements and down-scale on physical office space. It is therefore expected that various hybrid work forms may remain in place for an extended duration, even after the pandemic is over [63, 64].
A second set of findings is that patterns of smartphone usage were closely associated with sleep timing and physical activity. E-device use, especially around bedtime, can disrupt sleep by displacing time allocated to sleep, increasing pre-sleep arousal, and suppressing circadian sleep drive [65]. The use of objective smartphone tracking allowed us to extract detailed temporal profiles of phone usage. Cluster analyses confirmed that heavier phone usage around bedtime was associated with later sleep and wake timing. These associations were most pronounced after reopening. Furthermore, heavier pre-bedtime smartphone usage was associated with lesser physical activity, both during lockdown and after reopening. From these findings it seems that heavy smartphone use is indeed associated with unfavorable health behaviors. When looking at self-rated wellbeing indicators, however, more phone usage was not necessarily associated with stronger negative outcomes. Individuals who had heavy phone usage did not report more stress or worse mood than those who had only light usage. Furthermore, some indications were found that individuals with intermediate levels of pre-bedtime phone usage reported slightly better mood, and lesser loneliness compared to light phone users.
While there has been much focus on the negative aspects of smartphone use, the smartphone can also be a vital tool in accessing information and maintaining social connectivity when communities are physically separated [34, 66–69]. Moreover, access to online entertainment can help people to wind down after a day of work/study [70]. The finding that, prior to bedtime, time spent on social media, video, and browser apps most strongly increased, indeed suggests such leisurely usage. In practice, it is likely that the reliance on digital devices will only grow in the near future. In our data, we did not observe a reduction in phone usage after reopening (despite marked increases upon initial lockdown instatement, as documented in prior research) [20, 21, 23]. To arrive at effective and practicable recommendations, it seems necessary to take a more balanced approached, weighing both the potential positive and negative effects [71].
Several limitations of the current study should be noted. First of all, data were based on a convenience sample of University students and employees. As such, results may not be representative for the wider population. Our study sample contained a relatively high percentage of students (60.6%), and relatively more women (69.2%) than men (30.8%). Furthermore, most participants were single or married without children (93.9%), precluding a comparison of effects between individuals with and without parental caregiving duties [72]. Importantly, although this study was initiated before the Singapore lockdown was announced, recruitment only started after instatement of the lockdown. Therefore, no pre-lockdown data were collected in this study for comparison. However, highly consistent lockdown-related lifestyle changes have been documented across different populations and countries [2, 16, 17, 73]. Prior studies in Singapore confirm these lockdown effects in wider samples of working adults and children [11, 35]. It should further be noted that no a priori sample size estimation could be done, as no prior studies were available to base power calculations on. Instead, sample size was determined by the number of Oura sleep tracking devices that were available for distribution for this study. As the pandemic situation has naturally impeded data collection from human subjects, much research has relied on survey data [74]. By combining objective tracking of activity, sleep and phone data, with participants’ self-report through EMA, the current study provides longitudinal insights, while minimizing reporting biases associated with (retrospective) surveys responses [19, 75–78]
Conclusion
The current data shows a complex pattern of changes in lifestyle and wellbeing after lockdown measures were lifted involving shorter and earlier sleep, increased physical activity but also increased stress. These changes were in opposite direction as shifts observed upon lockdown onset, as reported elsewhere. The addition of daily work location and phone use data uncovered persistent, heterogenous effects of residual mobility restrictions on sleep, activity, and wellbeing underscoring the utility of multimodal longitudinal behavioral data in informing strategies to better cope with an extended pandemic and its aftermath.
Supplementary Material
Acknowledgments
The authors like to thank Andrew Dicom for assistance in participant recruitment and communication during the recruitment phase of the study.
Funding
This work was supported by a grant from the National Medical Research Council Singapore (STaR May2019-001) and Support Funds for the Centre for Sleep and Cognition awarded to Michael W. L. Chee.
Disclosure Statement
Financial disclosure: The authors have no financial conflicts of interest to declare.
Nonfinancial disclosure: Michael W. L. Chee sponsored the development of the Z4IP Ecological Momentary Assessment App, and is an advisor for QuantActions Ltd, Lausanne, Switzerland. Software and data collection services from QuantActions were used to monitor smartphone activity. Stijn A. A. Massar, Alyssa S. C. Ng, Chun Siong Soon, Ju Lynn Ong, Xin Yu Chua, Nicholas I. Y. N. Chee, and Tih Shih Lee have no competing interests to declare.
Material and Data Availability
Questionnaires and data used in this report are deposited in the Open Science Framework repository (https://osf.io/f43h6/).
References
- 1. Pépin JL, et al. . Wearable activity trackers for monitoring adherence to home confinement during the COVID-19 pandemic worldwide: data aggregation and analysis. J Med Internet Res. 2020;22(6):e19787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tison GH, et al. . Worldwide effect of COVID-19 on physical activity: a descriptive study. Ann Intern Med. 2020;173(9):767–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stockwell S, et al. . Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc Med. 2021;7(1):e000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Killgore WDS, et al. . Three months of loneliness during the COVID-19 lockdown. Psychiatry Res. 2020;293:113392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varma P, et al. . Younger people are more vulnerable to stress, anxiety and depression during COVID-19 pandemic: a global cross-sectional survey. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang Y, et al. . Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. 2020;288:112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barrero JM, et al. . 60 Million Fewer Commuting Hours per Day: How Americans Use Time Saved by Working from Home. University of Chicago, Becker Friedman Institute for Economics; Working Paper No. 2020-132; 2020. [Google Scholar]
- 8. Blume C, et al. . Effects of the COVID-19 lockdown on human sleep and rest-activity rhythms. Curr Biol.2020;30(14):R795–R797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cellini N, et al. . Changes in sleep timing and subjective sleep quality during the COVID-19 lockdown in Italy and Belgium: age, gender and working status as modulating factors. Sleep Med. 2021;77:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wright KP Jr, et al. . Sleep in university students prior to and during COVID-19 Stay-at-Home orders. Curr Biol. 2020;30(14):R797–R798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ong JL, et al.. COVID-19-related mobility reduction: heterogenous effects on sleep and physical activity rhythms. Sleep. 2021;44(2). doi: 10.1093/sleep/zsaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rezaei N, et al. . Changes in sleep duration, timing, and variability during the COVID-19 pandemic: large-scale Fitbit data from 6 major US cities. Sleep Health. 2021;7(3):303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franceschini C, et al. . Poor sleep quality and its consequences on mental health during the COVID-19 lockdown in Italy. Front Psychol. 2020;11:574475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinto J, et al. . Sleep quality in times of Covid-19 pandemic. Sleep Med. 2020;74:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alzueta E, et al. . How the COVID-19 pandemic has changed our lives: a study of psychological correlates across 59 countries. J Clin Psychol. 2021;77(3):556–570. [DOI] [PubMed] [Google Scholar]
- 16. Yuksel D, et al. . Sleeping when the world locks down: correlates of sleep health during the COVID-19 pandemic across 59 countries. Sleep Health. 2021;7(2):134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robbins R, et al. . Estimated sleep duration before and during the COVID-19 pandemic in major metropolitan areas on different continents: observational study of smartphone app data. J Med Internet Res. 2021;23(2):e20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ong JL, et al. . A longitudinal analysis of COVID-19 lockdown stringency on sleep and resting heart rate measures across 20 countries. Sci Rep. 2021;11(1):14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hodes LN, Thomas KGF. Smartphone screen time: inaccuracy of self-reports and influence of psychological and contextual factors. Comput Human Behav.2021;115:106616. [Google Scholar]
- 20. Sun S, et al. ; RADAR-CNS Consortium. Using smartphones and wearable devices to monitor behavioral changes during COVID-19. J Med Internet Res. 2020;22(9):e19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sañudo B, et al. . Objectively-assessed physical activity, sedentary behavior, smartphone use, and sleep patterns pre- and during-COVID-19 quarantine in young adults from Spain. Sustainability. 2020;12(15):5890. [Google Scholar]
- 22. Vargo D, et al. . Digital technology use during COVID-19 pandemic: a rapid review. Hum Behav Emerg Technol. 2021;3(1):13–24. [Google Scholar]
- 23. Giuntella O, et al. . Lifestyle and mental health disruptions during COVID-19. Proc Natl Acad Sci USA. 2021;118(9):e2016632118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huckins JF, et al. . Mental health and behavior of college students during the early phases of the COVID-19 pandemic: longitudinal smartphone and ecological momentary assessment study. J Med Internet Res. 2020;22(6):e20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith L, et al. . The association between screen time and mental health during COVID-19: a cross sectional study. Psychiatry Res. 2020;292:113333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Czeisler MÉ, et al.. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic—United States, June 24–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han E, et al. . Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet. 2020;396(10261):1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hale T, et al. . A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat Hum Behav. 2021;5(4):529–538. [DOI] [PubMed] [Google Scholar]
- 29. Skegg D, et al. . Future scenarios for the COVID-19 pandemic. Lancet. 2021;397(10276):777–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scudellari M. How the pandemic might play out in 2021 and beyond. Nature. 2020;584(7819):22–25. [DOI] [PubMed] [Google Scholar]
- 31. Ministry of Health Singapore. Circuit Breaker to Minimise Further Spread of Covid-19.https://www.moh.gov.sg/news-highlights/details/circuit-breaker-to-minimise-further-spread-of-covid-19. Accessed September 9, 2021.
- 32. Long VJE, et al. . Behavioural changes during the COVID-19 pandemic: results of a nationwide survey in Singapore. Ann Acad Med Singap. 2021;50(3):222–231. [PubMed] [Google Scholar]
- 33. Cheng TC, et al. . The Impact of COVID-19 on Subjective Well-Being: Evidence from Singapore2020. Bonn, Germany: IZA Discussion Papers, IZA – Institute of Labor Economics. [Google Scholar]
- 34. Liu JCJ, et al. . The relation between official WhatsApp-distributed COVID-19 news exposure and psychological symptoms: cross-sectional survey study. J Med Internet Res. 2020;22(9):e22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lim MTC, et al. . School closure during the coronavirus disease 2019 (COVID-19) pandemic – impact on children’s sleep. Sleep Med. 2021;78:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ministry of Health Singapore. End of Circuit Breaker, Phased Approach to Resuming Activities Safely . https://www.moh.gov.sg/news-highlights/details/end-of-circuit-breaker-phased-approach-to-resuming-activities-safely. Accessed September 9, 2021.
- 37. Gov.sg. Moving into Phase 2: What Activities Can Resume . https://www.gov.sg/article/moving-into-phase-2-what-activities-can-resume. Accessed September 9, 2021.
- 38. Ministry of Health Singapore. Moving Into Phase Three of Re-Opening.https://www.moh.gov.sg/news-highlights/details/moving-into-phase-three-of-re-opening. Accessed September 9, 2021.
- 39. Korman M, et al. . COVID-19-mandated social restrictions unveil the impact of social time pressure on sleep and body clock. Sci Rep. 2020;10(1):22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leone MJ, et al. . Effects of lockdown on human sleep and chronotype during the COVID-19 pandemic. Curr Biol. 2020;30(16):R930–R931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salfi F, et al.. Changes of evening exposure to electronic devices during the COVID-19 lockdown affect the time course of sleep disturbances. Sleep. 2021;44(9). doi: 10.1093/sleep/zsab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghosh A. Linking elementary properties of the human brain to the behaviour captured on touchscreen smartphones. In: Montag C, Reuter M, eds. Internet Addiction: Neuroscientific Approaches and Therapeutical Implications Including Smartphone Addiction. Cham, Switzerland: Springer International Publishing; 2017:373–381. [Google Scholar]
- 43. de Zambotti M, et al.. The sleep of the ring: comparison of the OURA sleep tracker against polysomnography. Behav Sleep Med. 2019;17(2):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stone JD, et al. . Evaluations of commercial sleep technologies for objective monitoring during routine sleeping conditions. Nat Sci Sleep. 2020;12:821–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roberts DM, et al. . Detecting sleep using heart rate and motion data from multisensor consumer-grade wearables, relative to wrist actigraphy and polysomnography. Sleep. 2020;43(7). doi: 10.1093/sleep/zsaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mehrabadi MA, et al.. Sleep tracking of a commercially available smart ring and smartwatch against medical-grade actigraphy in everyday settings: instrument validation study. JMIR mHealth and uHealth. 2020;8(11): e20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chee NI, et al.. Multi-night validation of a sleep tracking ring in adolescents compared with a research actigraph and polysomnography. Nat Sci Sleep. 2021;13:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Massar SAA, et al.. Trait-like nocturnal sleep behavior identified by combining wearable, phone-use, and self-report data. npj Digit Med. 2021;4(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sliwinski MJ, et al. . Reliability and validity of ambulatory cognitive assessments. Assessment. 2018;25(1):14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Benjamini Y, et al. . Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc Ser B Methodol. 1995;57(1):289–300. [Google Scholar]
- 51. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York, NY: Routledge Academic; 1988. [Google Scholar]
- 52. Conroy DA, et al. . The effects of COVID-19 stay-at-home order on sleep, health, and working patterns: a survey study of US health care workers. J Clin Sleep Med. 2021;17(2):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ni MY, et al. . Mental health, risk factors, and social media use during the COVID-19 epidemic and cordon sanitaire among the community and health professionals in Wuhan, China: cross-sectional survey. JMIR Ment Health. 2020;7(5):e19009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leger D, et al. . Poor sleep associated with overuse of media during the COVID-19 lockdown. Sleep. 2020;43(10). doi: 10.1093/sleep/zsaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cellini N, et al. . Changes in sleep pattern, sense of time and digital media use during COVID-19 lockdown in Italy. J Sleep Res. 2020;29(4):e13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McCarthy H, et al. . Physical activity behavior before, during, and after COVID-19 restrictions: longitudinal smartphone-tracking study of adults in the United Kingdom. J Med Internet Res. 2021;23(2):e23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. gov.sg. Ending Circuit Breaker: Phased Approach to Resuming Activities Safely. https://www.gov.sg/article/ending-circuit-breaker-phased-approach-to-resuming-activities-safely. Accessed September 9, 2021.
- 58. Ministry of Health Singapore. National Population Health Survey 2019. Singapore: Epidemiology & Disease Control Division and Policy, Research & Surveillance Group, Ministry of Health and Health Promotion Board; 2019:17– 25. https://www.hpb.gov.sg/docs/default-source/default-document-library/national-population-health-survey-2019.pdf. [Google Scholar]
- 59. MacDonald JM, et al. . The effect of light rail transit on body mass index and physical activity. Am J Prev Med. 2010;39(2):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rissel C, et al. . Physical activity associated with public transport use – a review and modelling of potential benefits. Int J Environ Res Public Health. 2012;9(7):2454–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Skeldon AC, et al. . The effects of self-selected light-dark cycles and social constraints on human sleep and circadian timing: a modeling approach. Sci Rep. 2017;7:45158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Salfi F, et al. . Sleeping under the waves: a longitudinal study across the contagion peaks of the COVID-19 pandemic in Italy. J Sleep Res. 2021;30(5):e13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lund S, et al.. The Future of Work After COVID-19. McKinsey Global Institute; 2021. [Google Scholar]
- 64. Barrero JM, et al. . Why Working From Home Will Stick. National Bureau of Economic Research Working Paper Series No. 28731; 2021. [Google Scholar]
- 65. Cain N, et al. . Electronic media use and sleep in school-aged children and adolescents: a review. Sleep Med. 2010;11(8):735–742. [DOI] [PubMed] [Google Scholar]
- 66. Cho J. Roles of smartphone app use in improving social capital and reducing social isolation. Cyberpsychol Behav Soc Netw. 2015;18(6):350–355. [DOI] [PubMed] [Google Scholar]
- 67. David ME, et al. . Smartphone use during the COVID-19 pandemic: social versus physical distancing. Int J Environ Res Public Health. 2021;18(3):1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stuart J, et al. . Online social connection as a buffer of health anxiety and isolation during COVID-19. Cyberpsychol Behav Soc Netw. 2021;24(8):521–525. [DOI] [PubMed] [Google Scholar]
- 69. Drouin M, et al. . how parents and their children used social media and technology at the beginning of the COVID-19 pandemic and associations with anxiety. Cyberpsychol Behav Soc Netw. 2020;23(11):727–736. [DOI] [PubMed] [Google Scholar]
- 70. Rodgers S, et al. . Managing stress, sleep and technologies. In: Proceedings of the 28th Australian Conference on Computer-Human Interaction – OzCHI ‘16; 2016.
- 71. Orben A, et al. . Only holistic and iterative change will fix digital technology research. Psychol Inq.2020;31(3): 235–241. [Google Scholar]
- 72. Davidson B, et al. . Risk and resilience of well-being in caregivers of young children in response to the COVID-19 pandemic. Transl Behav Med. 2021;11(2):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee PH, et al. . Sleep pattern in the US and 16 European countries during the COVID-19 outbreak using crowdsourced smartphone data. Eur J Public Health. 2021;31(1):23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aschwanden C. How COVID is changing the study of human behaviour. Nature. 2021;593(7859):331–333. [DOI] [PubMed] [Google Scholar]
- 75. Hipp L, et al. . Problems and pitfalls of retrospective survey questions in COVID-19 studies. Surv Res Meth. 2020;14(2):1109–1145. [Google Scholar]
- 76. Gao C, et al. . Sleep health early in the coronavirus disease 2019 (COVID-19) outbreak in the United States: integrating longitudinal, cross-sectional, and retrospective recall data. Sleep Med. 2020;73:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Parry DA, et al. . A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use. Nat Hum Behav. 2021. [DOI] [PubMed] [Google Scholar]
- 78. Burnell K, et al. . Associations between Self-Reports and Device-Reports of Social Networking Site Use: An Application of the Truth and Bias Model. Commun Methods Meas. 2021;15(2):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.