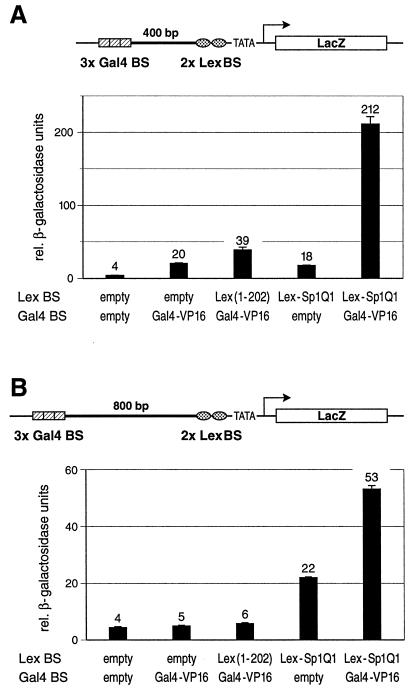
FIG. 3.
The herpes simplex viral activator VP16 synergizes with a proximal glutamine-rich activation domain of Sp1 over long distances in yeast. (A) The potent activation domain of the herpes simplex transactivator VP16 fused to the Gal4 DBD (Gal4-VP16) was tested for its ability to stimulate gene expression from remote Gal4 binding sites (Gal4 BS). These binding sites were separated from the proximal Lex binding sites (Lex BS) by a 400-bp spacer sequence. A schematic drawing of the chromosomal reporter construct is indicated above the graph. Distal Gal4-VP16 or proximal Sp1Q1 fused to LexA (Lex-Sp1Q1) alone activated to comparable low levels. Combination of both resulted in a more-than-tenfold increase of reporter gene transcription as compared to the effect of each activator tested on its own. (B) Over the very long distance of 800 bp, the Gal4-VP16 transactivator did not stimulate gene expression at all when tested either alone or with the proximal, transcriptionally inert LexA. However, in combination with the proximally bound glutamine-rich domain of Sp1 (Lex-Sp1Q1), Gal4-VP16 strongly contributed to gene expression, i.e., under these conditions, the enhancer-bound activator is strictly dependent on the presence of a promoter-proximal glutamine-rich activation domain.