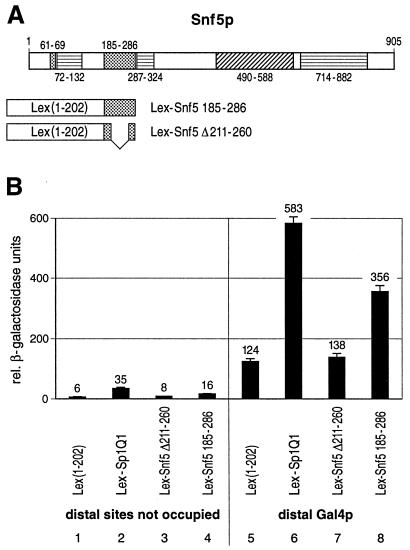
FIG. 6.
The glutamine-rich domain of Snf5p activates transcription in yeast. (A) Schematic drawing of the 905-amino-acid-long Snf5p factor which contains an acidic region (amino acids 490 to 588), three proline-rich domains (amino acids 72 to 132, 287 to 324, and 714 to 882), and two glutamine-rich domains (amino acids 61 to 69 and 185 to 286). The boundary amino acid positions of the corresponding domains are indicated. The larger glutamine-rich domain of Snf5p (amino acids 185 to 286) (Lex-Snf5 185-286) as well as a deletion mutation (deletion of amino acids 211 to 260) of this glutamine-rich domain (Lex-Snf5 Δ211-260) were fused to the Lex DBD. (B) Quantitative β-galactosidase assay. The glutamine-rich domain of Snf5p stimulated LacZ expression when bound proximally to the TATA box (lane 4) and synergized with a distal Gal4p activator (lane 8). For comparison, the stronger glutamine-rich activation domain of Sp1 (Lex-Sp1Q1) was used (lanes 2 and 6). The 50-amino-acid deletion abolished the transactivation potential of the Snf5p glutamine-rich domain (lane 3) and hence the synergism with the distal Gal4p activator (lane 7).