Fig. 2.
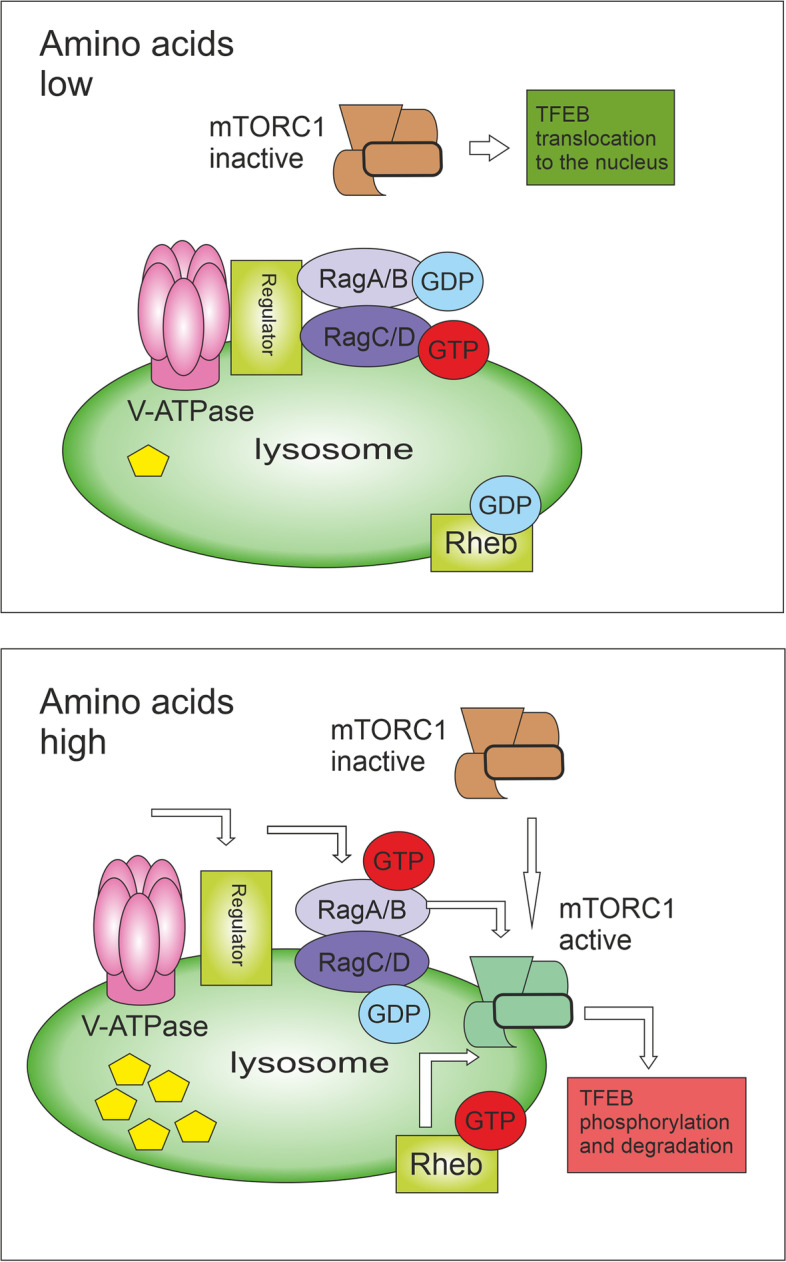
Amino acid-based mTORC1 activation. By amino acid starvation, the inactive V-ATPase-Ragulator complex is unable to activate Rag GTPases on the lysosomal surface, thus mTORC1 is not recruited to the lysosome. The inactivation of mTORC1 leads to rapid translocation of transcription factors TFEB and TFE3 to the nucleus. Active TFEB upregulates the expression of lysosomal genes and critical regulators of autophagy. By amino acid abundancy, the V-ATPase undergoes conformational changes leading to the activation of Regulator, which in turn promotes the Rag heterodimer activation. Active Rag heterodimer (RagA/B(GTP)-RagC/D(GDP)) then recruits mTORC1 to the lysosomal surface where Rheb is present. Rheb can directly bind and activate mTORC1. TFEB is recruited on the lysosomal membrane, phosphorylated by active mTORC1, and then degraded by the proteasome
