FIG 1.
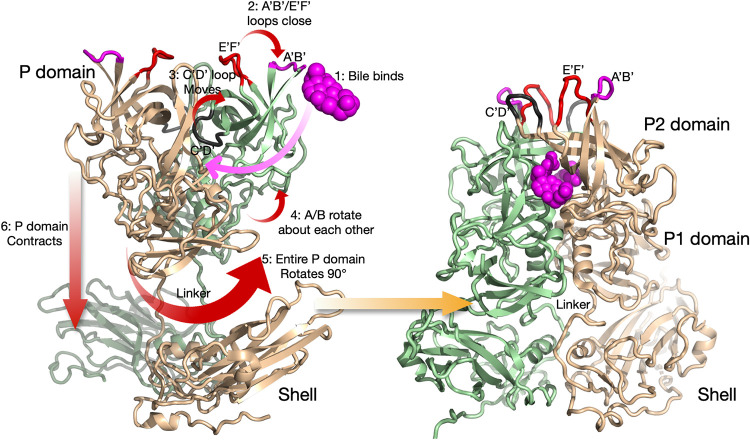
Summary of the conformational changes in MNV upon bile binding (18). Shown here is a capsid dimer, with the two subunits colored blue and tan. The P domain is highly flexible and in structural equilibrium in solution. The binding of bile likely drives the structural equilibrium in the following manner. Bile binds between the P domain dimers (step 1), and this is likely facilitated by the flexibility of the C′-D′ and A′-B′/E′-F′ loops. As bile binds, the equilibrium is pushed toward a closed conformation for the A′-B′ and E′-F′ loops (step 2), and the C′-D′ loop moves up to stabilize that conformation (step 3). Likely related to the C′-D′ loop movement, the P domains in the dimer rotate slightly about each other (step 4), and this new surface at the base is now compatible with that of the top of the shell. This leads to an ∼90° rotation of the entire P domain (step 5) and contraction of the P domain onto the shell (step 6). The contracted structure with the P domain in the closed conformation, after all of the conformational changes have occurred, is shown on the right.