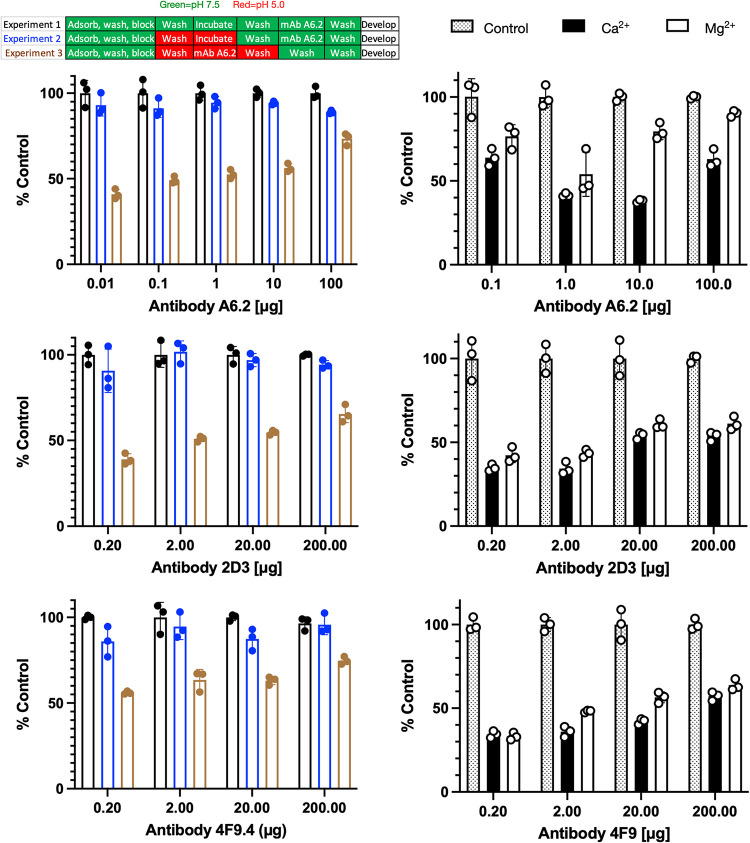
FIG 6.
Effects of pH and metals on MAb A6.2, 2D3, and 4F9 binding to infectious particles of MNV. Shown here is the effect of low pH on A6.2 binding using the same citrate/phosphate buffers used for the structural work. The design of the experiment is outlined in the bar figure shown at the top. For experiment 1, the pH 7.5 buffer was used throughout the experiment. For experiment 2, the virus was adsorbed to the plate, treated with pH 5.0 buffer for 1 h, and washed with pH 7.5 buffer, and the experiment was completed in the same buffer. Loss of signal during this process would imply irreversible denaturation of MNV at low pH. Finally, in experiment 3, MNV was washed and incubated with pH 5.0 buffer immediately before, during, and after incubation with antibody. Loss of signal, compared to experiment 2, demonstrates that all three antibodies do not bind as well to the pH 5 conformation. Since the pH 5.0 structure is nearly identical to the X-ray structure in the presence of Mg2+ (12), ELISAs were performed with metals added during the incubation with MAbs A6.2, 2D3, and 4F9. As shown here, both calcium and magnesium significantly blocked antibody binding. Therefore, bile (18), low pH, and metals all decrease the affinity of all three antibodies to the virion by pushing the structural equilibrium to the closed conformation.