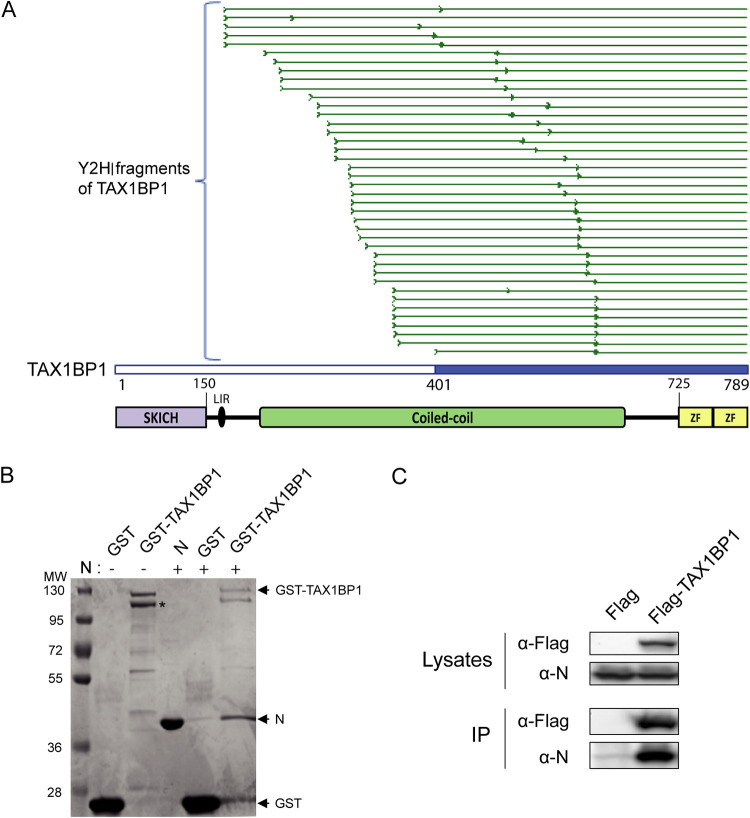
FIG 1.
Identification and validation of the TAX1BP1-N interaction. (A) Multiple-sequence alignment of sequencing reads obtained from the 40 yeast colonies matching TAXBP1. As the cDNA library used in the screen was built by oligo(dT) priming, TAX1BP1 fragments captured in the screen extend from the beginning of the sequencing reads (thick green line) to the end of the TAX1BP1 sequence. The shortest TAX1BP1 fragment captured with Nmono is depicted in blue. Below the alignment, a scheme of the TAX1BP1 structural organization is presented, with numbers indicating residues of TAX1B1P1: the SKIP carboxyl homology domain (SKICH), the LC3-interacting region (LIR), central coiled coils constituting the oligomerization domain, and the two C-terminal zinc fingers (ZF). (B) Validation of the N-TAX1BP1 interaction by GST pulldown with recombinant proteins. GST and GST-TAX1BP1 proteins were purified on glutathione-Sepharose beads and incubated in the presence of recombinant N protein, and interactions were analyzed by SDS-PAGE and Coomassie blue staining. The asterisks indicate the product of degradation of GST-TAX1BP1 corresponding to the deletion of the C-terminal domain. Molecular masses (MW) corresponding to the ladder’s bands are indicated. (C) Western blot analysis of the TAX1BP1-N interaction after an immunoprecipitation assay. Cells were transiently transfected with constructs allowing the expression of the Flag tag alone or the Flag-TAX1BP1 fusion protein with N protein. Immunoprecipitations (IP) were performed with an anti-Flag antibody.