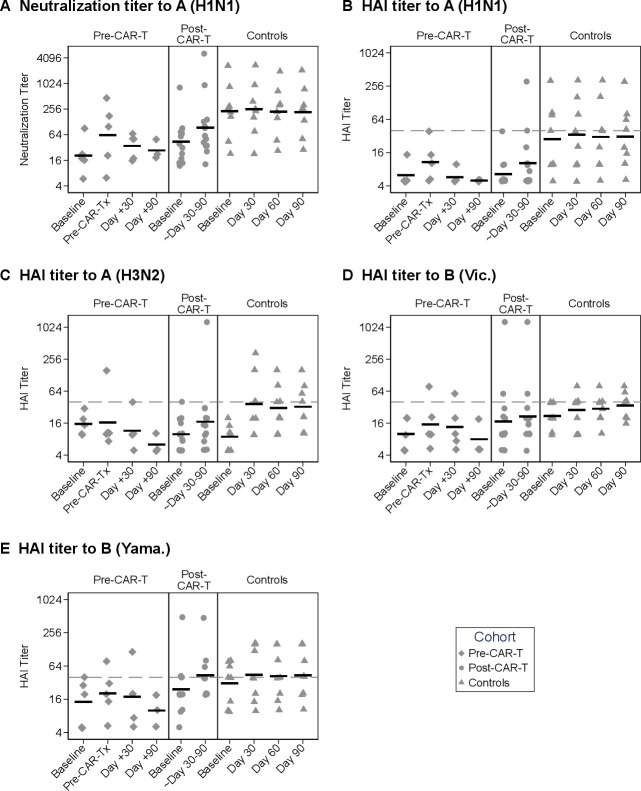
Figure 1.
Summary of longitudinal influenza antibody kinetics and geometric mean titers (GMTs). Individual titer results are plotted for each sample collection time point for the pre-CAR-T, post-CAR-T, and control cohort (from left to right in each panel). (A) Neutralization titers to A(H1N1) and (B) HAI titers to A(H1N1), (C) A(H3N2), (D) B(Victoria), and (E) B(Yamagata) are shown. A value of half of the lower limit of detection (LOD) was assigned for values below the LOD (LODs are detailed in the Methods). Data have been jittered to allow viewing of overlapping values. Horizontal bars represent GMT. Symbols on or above the dashed horizontal line represent HAI titers ≥40. Baseline titers to A(H1N1) were significantly lower in the CAR-T cohorts when compared with the control cohort (neutralization assay: pre-CAR-T vs controls, p=0.01; post-CAR-T vs controls, p=0.02. HAI assay: pre-CAR-T vs controls, p=0.02; post-CAR-T vs controls, p=0.004; based on Dunn’s test with the Holm stepwise procedure for multiple comparisons). There were no significant differences in baseline titers between cohorts based on the HAI assay to A(H3N2), B(Victoria), or B(Yamagata) (Kruskal-Wallis, p=0.21, p=0.17 and p=0.36, respectively). CAR-T-cell, chimeric antigen receptor-modified T cell; HAI, hemagglutination inhibition.