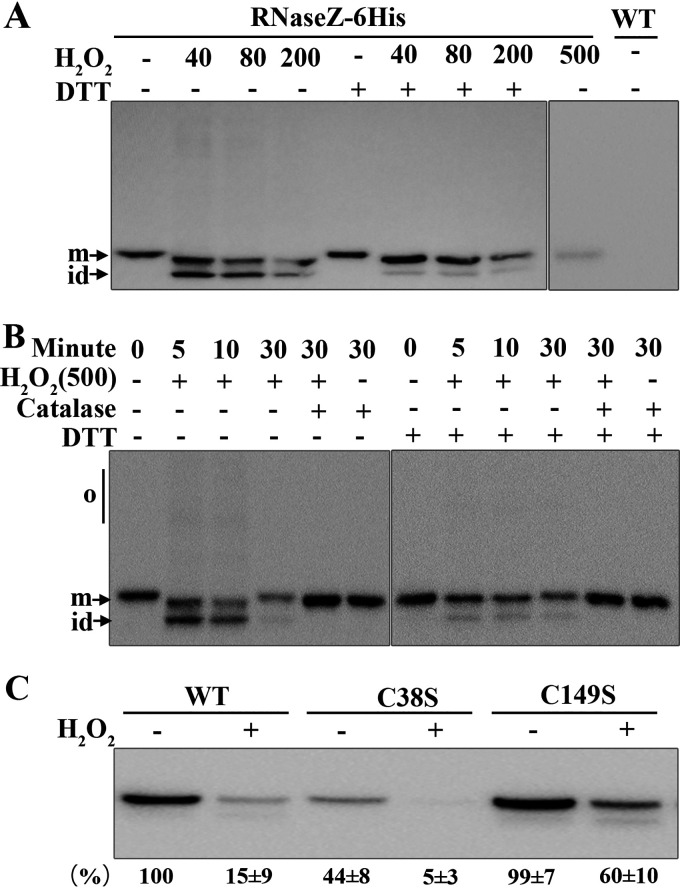
FIG 3.
Examination of disulfide linkages and degradation of So-RNaseZ in H2O2-treated S. oligofermentans. (A and B) Mid-exponential-phase anaerobically grown RNaseZ-6×His cells, which carry a So-RNaseZ C-terminal fusion with a 6×His tag, were 30 min treated without (−) or with 40, 80, 200, and 500 μM H2O2 (A), or treated with 500 μM H2O2 for different time periods and concomitantly with 1 KU catalase or not (B). Cells were then lysed, and one aliquot was reduced with 10 mM DTT. All samples were run on 10% nonreducing SDS-PAGE gels, and So-RNaseZ was examined by Western blotting using anti-His antibody. The wild-type strain (WT) was included as a negative control. o, oligomers; m, monomer; and id, intramolecular disulfide-linked monomer. All experiments were repeated three times, and representative images are shown. (C) Wild-type So-RNaseZ6×His (WT) and C38S- and C149S-mutated So-RNaseZ6×His complemented strains were treated with 500 μM H2O2 for 30 min, and the cellular levels of So-RNaseZ were examined by Western blotting using anti-His antibody. Band intensities of the So-RNaseZ protein were measured using Image J and expressed as percentages of WT cell lysate without treatment with H2O2. The experiments were repeated three times, and the averages ± standard deviation (SD) are shown below a representative image.