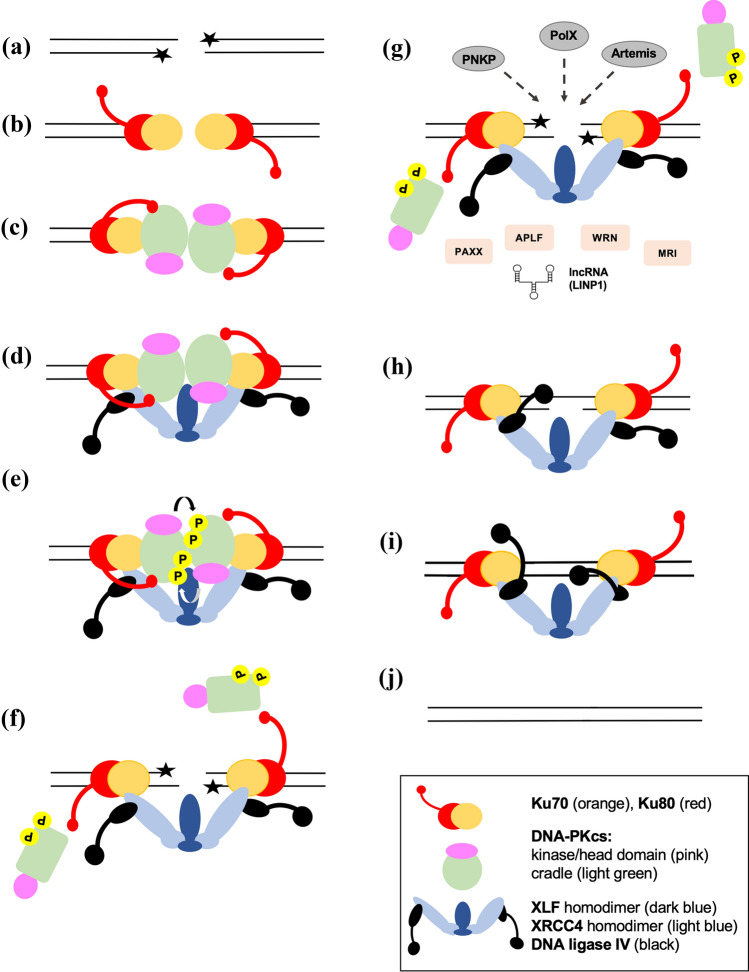
Fig. 2.
Model for NHEJ. A IR induces DSBs, often with damaged ends that contain non-ligatable end groups (stars). B A Ku heterodimer (composed of Ku70, orange; Ku80, red) binds to each end of the DSB and C recruits DNA-PKcs (shown with head /kinase-FAT-FATC domain in pink and circular cradle in green) to form a synaptic complex. D The Ku complex also recruits a single homodimer of XLF (dark blue) that interacts via its head domain with two XRCC4 homodimers (light blue). The coiled-coil domain of XRCC4 interacts with tandem BRCT domains in the C-terminal region of DNA ligase IV (black). This forms a long-range synaptic complex as reported in Chen et al. (2021a). E Autophosphorylation of DNA-PKcs at ABCDE and possibly other sites, likely in trans leads to a conformational change that causes release of the DSB ends by DNA-PKcs. F autophosphorylated DNA-PKcs dissociates from the complex providing access to processing enzymes such as PNKP, Artemis and DNA polymerases mu and lambda which remove non-ligatable end groups and fill in gaps (G). H and I each DNA ligase IV is tethered to an XRCC4 homodimer through its C-terminal BRCT domains while the N-terminal catalytic domain is attached via a flexible linker region. This allows the catalytic domains to access the DSB ends while Ligase IV remains tethered to the synaptic complex. Each DNA Ligase IV repairs a single-strand break so that the two breaks are repaired sequentially. J The break is repaired restoring genome integrity. G shows additional NHEJ factors such as PAXX, APLF, WRN, Cyren/MRI and long non-coding RNA which may also be involved. The precise order of recruitment and dissociation of the NHEJ proteins is unknown