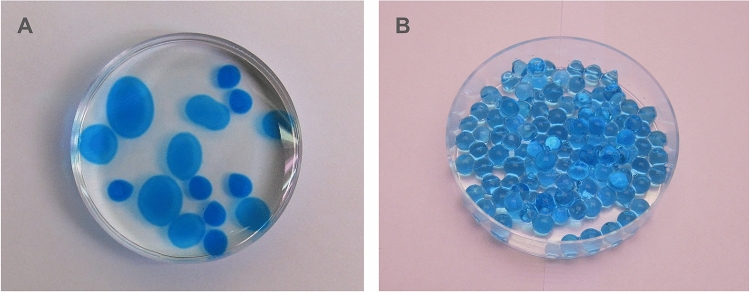
Fig. 1.
Ca2+-alginate capsules a and beads b inside a petri dish (diam 60 mm). The capsules are of different sizes because they were made with different numbers of droplets of a CaCl2∙2H2O solution applied to a sodium alginate solution (Kim et al. 2015). Note that each capsule shell surrounds a liquid core. In contrast, the Ca2+-alginate beads were solid and were produced by dropping single droplets of sodium alginate solution into a CaCl2·2H2O solution. All beads are of the same size because for each drop cross-linking occurs immediately due to excessive Ca2+ ions, preventing the formation of a liquid core.
Figure 1a was reused from Kim et al. 2015 with permission of the editor