ABSTRACT
Objectives
The effects of trigger point dry needling (TDN) on myofascial trigger points (MTP) in Achilles tendinopathy (AT) are unknown. We conducted a study to test the feasibility of a large randomized controlled trial (RCT) to compare the effects of TDN to MT and exercise in a patient population with AT.
Methods
Twenty-two subjects were randomly assigned to a control (MT+Ex) or experimental group (TDN+MT+Ex) and completed eight treatment sessions over 4 weeks with follow up at 3 months. TDN was performed to MTPs in the gastrocnemius, soleus or tibialis posterior each session. The same MT and exercise program was conducted in both groups.
Results
Two of three criteria for feasibility were met. The attrition rate at 4-week and 3-month follow-up was 18.1% and 68%, respectively. Significant differences (p < .05) reported for within group analysis for FAAM, NPRS, pain pressure threshold and strength in both groups at 4 weeks and 3 months. The GROC was significant for MT + Ex at 3 months. No between group differences were found. The MCID for the FAAM, GROC was surpassed in both groups at 4 weeks and 3 months and NPRS for the MT + Ex group at 4 weeks.
Discussion
A large RCT to investigate the effects of TDN on MTP in AT is not feasible without modifications due to low recruitment and high attrition rate. Modifications to study design should give consideration for closed or national health-care system for access to large patient populations and reduced financial burden to subjects.
Trial Registration
ClinicalTrials.gov identifier: NCT03261504F.
KEYWORDS: Trigger point dry needling, myofascial trigger points, feasibility, Achilles, eccentric
Introduction
Achilles tendinopathy is the most prevalent pathology to the Achilles tendon. [1] Tendinopathy is classified as degenerative disorder with several contributing factors leading to the development [2–4]. Midportion tendinopathy is the most common pathology of the Achilles tendon and estimated to account for 55–65% of all incidences, while insertional tendinopathy follows with 20–25%[1]. The incidence of Achilles rupture is rising in the US and abroad, with evidence indicating tendinopathy is present prior to the rupture and may increase the rate of occurrence[5]. Achilles tendinopathy affects people with a sedentary or active lifestyle [6–9].
Mechanical loading through eccentric strengthening can stimulate a healing response in tendons [10,11]. Treatment programs for Achilles tendinopathy including eccentric exercise have reported improved pain and function [12] but the healing rate and functional improvements are slow with gradual improvements over a span of 4–5 years [13–16]. Other treatment options including extracorporeal shockwave therapy, tendon needling, platelet-rich plasma or corticosteroid injections have either modest benefit or questionable efficacy [12,16–19].
Pain from myofascial trigger points (MTPs) can refer to distant regions [20]. Active MTPs in the gastrocnemius, soleus and tibialis posterior have been shown to refer pain to the posterior heel [21]. Renin-Ordine reports a significant improvement in posterior heel pain following soft tissue mobilization to the MTPs in the lower leg in patients with posterior heel pain. Treatment for MTP with trigger point dry needling (TDN) has been reported to be effective and the number of studies reporting beneficial effects of TDN to MTPs is rapidly growing for several diagnoses. [22–24] These results occur in much shorter timeframes than what has been reported for eccentric exercise and other interventions for Achilles tendinopathy [15,16,25]. There are no studies in the published literature that have investigated the effects of TDN for myofascial trigger points in Achilles tendinopathy.
Despite these reported benefits, there are still unanswered questions regarding TDN and the effectiveness compared to other interventions [26]. At present, there is questionable reliability for diagnosing MTPs with clinical examination procedures [27] as well as concerns related to the recommended screening examination for performing TDN [28]. Furthermore, there is inconsistency with the parameters for TDN procedures, the importance of the local twitch response to outcomes [29] and insufficient data to determine if superficial or deep needling is more effective for long-term benefits [30].
Given the prevalence of Achilles tendinopathy and slow progress with eccentric training, as well as the reported effectiveness of TDN, a large randomized controlled trial is warranted to investigate the effect of TDN on pain and functional outcomes, although the feasibility to conduct a large RCT for Achilles tendinopathy is questionable. Changes in the health-care delivery model and rising out-of-pocket costs for medical care may negatively affect the opportunities to recruit subjects for clinical research [31–35]. In addition, TDN is not included in the physical therapy practice act of all states, limiting the regions that data collection may occur. Barriers to subject participation including aversion to needles [36] and attrition rate are additional factors that can limit the total number of subjects completing a study. Therefore, this preliminary study was developed to assist in the determination of the feasibility of a large RCT investigating the effects of TDN on posterior heel pain in patients with Achilles tendinopathy.
The primary objectives of the current feasibility study were to report on subject recruitment and practicality of conducting this study design while maintaining normal operations in physical therapy clinics. These objectives include:
Report the access to participants with Achilles tendinopathy in private, outpatient physical therapy clinics.
Identify barriers for subject participation (injury severity, fear of needles, cost of care, time to complete study protocol and follow-up).
Describe the practicality of subject recruitment during normal clinic operations in physical therapy clinics.
The secondary objectives for this study were developed to describe the outcomes of this study and determine if the treatment protocol is worthwhile to include or redesign. These include:
Report participant’s adherence to treatment.
Report and describe any adverse responses to treatment.
Report the attrition rate with follow-up data collection at 4 weeks and 3 months.
Report within, and between group treatment effects.
The determination of the feasibility for a randomized controlled trial will be based on the supporting data for each of the primary and secondary objectives [37–39]. Analysis of the primary objectives will be qualitative, while the secondary objectives will be quantitative. The determination of the feasibility of the treatment protocol as described in the secondary objectives was adopted from a feasibility study reported by Tough et al. [37]. The treatment protocol for a randomized controlled trial will be feasible if 1) the 4-week attrition rate is less than 20%; 2) no adverse responses are reported; and 3) at least 75% of patients complete follow-up assessment data.
Methods
This feasibility study was conducted with a single-factor, pretest-posttest control group design. CONSORT guidelines with extension for randomized feasibility and pilot studies were followed for reporting the results of this study [40]. Consecutive patients presenting to data collection facilities were eligible to participate in the study. All data were collected in a total of seven private outpatient physical therapy clinics in North Carolina, Tennessee and Iowa by physical therapists. The primary investigator has over 20 years of clinical practice and over 10 years of experience performing TDN. There were seven additional physical therapists who contributed to the data collection. The clinical experience ranges from 2 to 20 years and all clinicians were certified to perform TDN for over 1 year at the time of data collection. All but one of the investigators were orthopedic clinical specialists and one was dual credentialed with orthopedic and sports.
The following conditions had to be met for the participants to be eligible to participate in the study:
Participants were between the ages of 18–70 years
Subjective report of the primary location of pain at any point along the Achilles tendon
Pain present ≥4 weeks
Positive Achilles palpation test
Positive Royal London test [2]
Decreased plantar flexion endurance test vs non-involved leg
The initial inclusion criteria were limited to non-insertional Achilles tendinopathy in the first year of data collection. However, the inclusion criteria were revised to include insertional Achilles tendinopathy due to low subject recruitment. The treatment protocol was not revised.
Patients with any of the following conditions were excluded from participating in the study:
fear of needles or unwilling to have needling performed due to fear or personal beliefs [41]
vascular or sensory disturbances in the lower leg which included but was not limited to injury to the nerve root or peripheral nerve in the affected lower leg, inflammatory diseases, bleeding or clotting disorders, lymphedema, peripheral vascular or peripheral arterial disease. Diabetes was included in this group due to the progressive changes to the sensation and circulation in the lower extremities.
recent infection
previous surgery to the foot/ankle
steroid by injection or transdermal delivery to the posterior heel within three months
full rupture of the Achilles tendon
pregnant or may be pregnant
Tampa Scale for Kinesiophobia >37 [15]
participants with a work-related injury insured by the bureau of worker’s compensation or involved in litigation related to injury of the lower leg, foot or ankle
The study was approved by the IRB at Nova Southeastern University and all patients provided informed consent prior to their participation. There was no compensation for the participants in this study.
Physical examination
On the first appointment, the physical therapist conducted a comprehensive examination to the lower leg, ankle and foot to verify the diagnosis of Achilles tendinopathy, presence of myofascial trigger points and study eligibility. A palpation exam was conducted for detecting myofascial trigger points as it has been found to be a reliable measure [37,38] but the controversy with reliability is noted [27, 42].
Data were collected at three intervals including baseline at the initial examination, at the conclusion of treatment at 4 weeks and follow-up at 3 months. The primary dependent variable was the Foot and Ankle Ability Measure (FAAM) while the secondary dependent variables were pain pressure threshold (PPT) measured with an algometer, global rating of change (GROC), numeric pain rating scale (NPRS) and strength. The FAAM is a self-report functional measure with subscales for ADL and sports. Only the ADL subscale was used in this study. This is a valid measure with an MCID of 8 points [43]. Pain threshold on the most painful site of the Achilles tendon was assessed with the FPK 20 PainTestTM algometer by Wagner Instruments [44]. The protocol for PPT measure was adopted from Renan-Ordine [21]. Pain pressure threshold measures were taken at the same location on the Achilles at the initial examination, at discharge as well as the 3-month follow-up examination. The GROC is a 15 point scale with an MCID of 3 points [45]. The NPRS is a valid measure with the MCID of 2 points [46]. The Tampa Scale of Kinesiophobia (TSK) was used for the exclusion criteria only with a cutoff score of 37 as recommended by Silbernagle [15]. Strength was measured with a single leg heel raise and is a reliable test for Achilles tendinopathy. [15,47]
Random group assignment
Data were collected in eight outpatient physical therapy clinics located in North Carolina, Tennessee and Iowa. The process for random assignment was the same for each clinic and done independent of each respective clinic. A blocked randomization procedure was used to balance the participants among groups with a computer-generated blocked randomization sequence [48]. Each participant was randomly assigned to the dry needling or exercise group according to the blocked randomization sequence. A concealed envelope with the group allocation was presented to the patient by an assistant to the evaluating physical therapist and opened in their presence.
Interventions
Participants began treatment on the day of the examination, immediately after randomization. If the participant was unable to complete the treatment on the day of the examination, then treatment began on the day of the next scheduled appointment. For these participants no interventions were performed, and no exercise was instructed for an independent home program until the participant returned for the next treatment. This session was their second visit to the clinic, but it was recorded as treatment number one.
Participants in both groups were treated in the clinic twice a week for 4 weeks for a total of eight treatment sessions. Each treatment session for Group 1 (MT + Ex) and group 2 (TDN + MT + Ex) included the interventions in order as shown in Figure 1. Exercises are shown in Figure 2. The treatment protocol was the same for participants with insertional and non-insertional tendinopathy.
Figure 1.
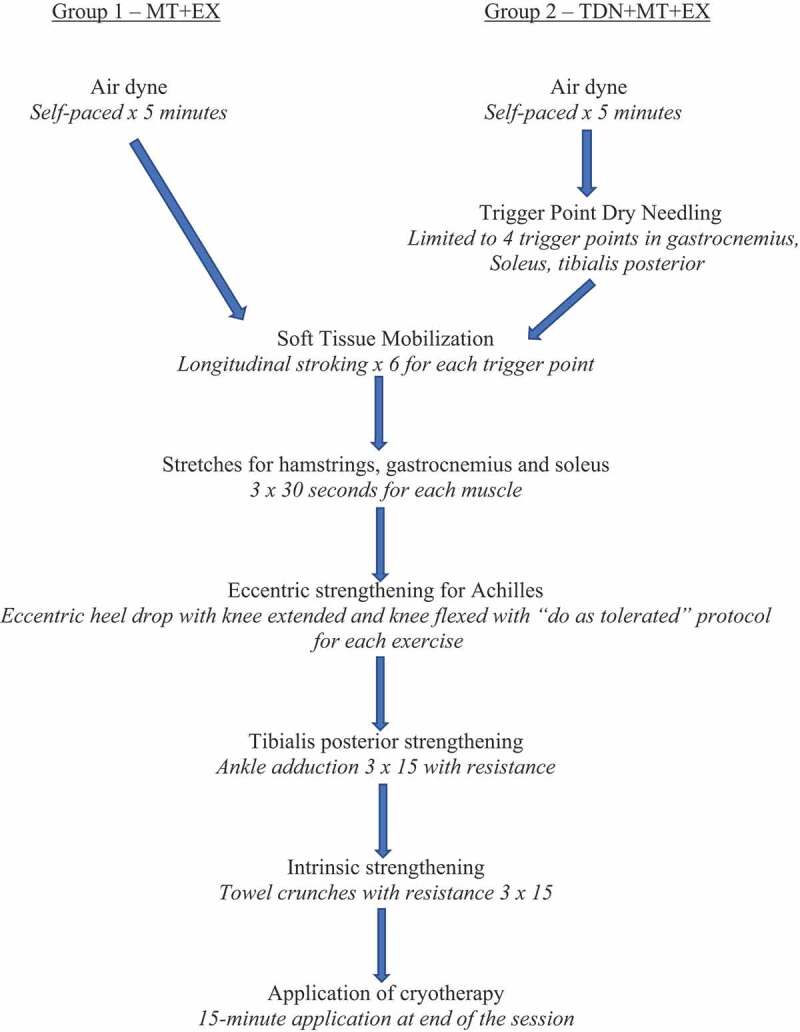
Sequence of Interventions for Each Treatment Session.
Figure 2.
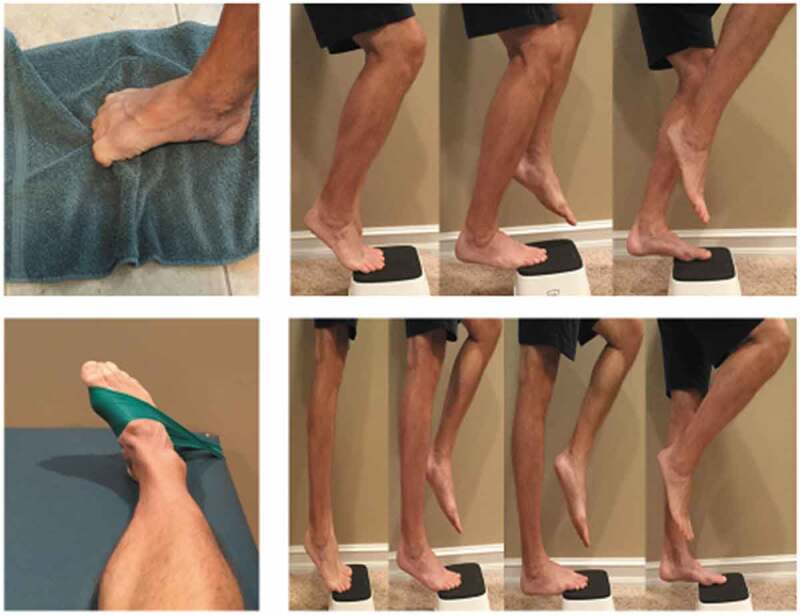
Exercises for both groups. Top Right: right leg eccentric lowering with knee flexed; Bottom Right: right leg eccentric heel lowering with knee extended; Bottom Left: resisted ankle adduction; Top Left: towel crunches.
The needling technique began with pistoning in a cone fashion around the trigger point. When a twitch was elicited, pistoning continued at that precise location until the twitch was exhausted. Once the cone was completed, the needle was rotated until there was sufficient needle grasp that would not allow the needle to be easily removed from the tissue. The needle remained until the grasp released and the needle was easily removed by the physical therapist.
A discharge examination was completed immediately following all interventions during the eighth treatment session. At this visit, participants completed the FAAM, PPT, TSK, GROC, NPRS and muscle endurance testing. Participants were instructed to continue with the exercise program independently and return for a follow-up examination at 3 months after the completion of the last treatment session. The 3-month follow-up examination included the FAAM, PPT, TSK, GROC, NPRS and strength tests.
Data analysis
The independent variable in this study was trigger point dry needling and time while the primary dependent variable was the FAAM and the secondary dependent variables were PPT, GROC, NPRS and strength utilizing the plantar flexion endurance test. Descriptive statistics were used to analyze data including participant adherence to treatment, attrition rate and baseline demographics. Categorical variables were calculated with frequency counts while continuous variables were calculated with measures of central tendency.
Friedman’s Test/Two Way Analysis of Variance by Ranks was conducted to assess effect size, changes in FAAM, PPT, GROC, NPRS and plantar flexion endurance measures for within group interaction and Mann Whitney U to assess between group analysis was performed at baseline, 4 weeks and 3 months [38]. The level of significance was set at p < .05. Data analysis was performed with SPSS 25. An intention to treat analysis was utilized for dropouts with the last value forward method being used to replace any missing data.
Results
Data collection began in May 2015 and terminated on August 1st, 2018. During this time 70 subjects were referred with Achilles tendinopathy and 34 subjects met the inclusion criteria. There were 36 subjects (51.6% of referrals) who were not eligible to participate in the study. There were 34 subjects (48.6%) who met the inclusion and 22 subjects (31.4%) who agreed to participate and provided informed consent. The flow diagram of subject recruitment and retention is described in Figure 3.
Figure 3.
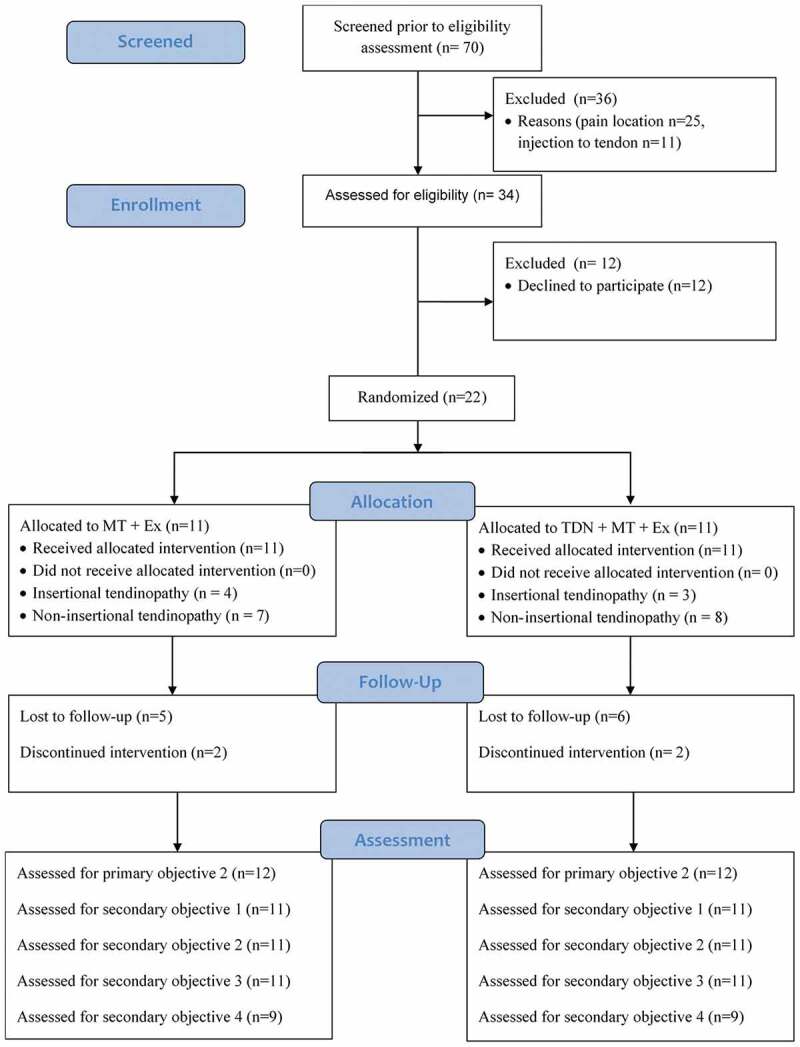
Consort flow diagram for subject recruitment and retention.
There were 12 patients (17.1% of referrals) eligible to participate in the study that declined. These patients declined participation due to averse to needle stick to painful trigger points (4), time commitment (2) and finances (6).
Each physical therapist collecting data was interviewed to determine if the study protocol was manageable during normal clinic operations. There was an average patient volume of 14 or more patients per 8-h day. All physical therapists rated the protocol to be manageable and were able to maintain their typical patient schedule without allotting additional time to complete the study protocol.
There were four subjects who voluntarily discontinued participation in the study resulting in an end of treatment drop out rate of 18.1%. Reasons for drop out included family emergency that resulted in extended delay upon return to treatment; time commitment due to family responsibilities; slow progress and returned to referring provider; one subject did not return for the final treatment session and discharge data collection despite reports of improved pain and functional activity tolerance. No serious adverse events were reported.
The attrition rate for the 3-month data collection was 61%. There was a total of seven subjects who returned for the 3-month follow-up while 11 subjects did not return across both groups. When factoring in the four subjects who dropped out of the study, the attrition rate increases to 68%.
Data analysis
A within group analysis for both groups included means and Friedman’s Two-Way Analysis of Variance by Ranks test were computed for all dependent variables. A mean of the demographic data on participants in each group is available in Table 1. The within group means, and significance for all tests and measures are provided in Table 2, while between group means are in Table 3. A statistically significant improvement with the within group analysis was achieved for all variables in both groups except for the GROC in the TDN+MT+Ex group.
Table 1.
Demographic Data.
| Group 1 (MT+Ex) |
Group 2 (TDN+MT+Ex) |
|||||
|---|---|---|---|---|---|---|
| NON | INS | Combined | NON | INS | Combined | |
| Age (yrs) | 49.4 | 41 | 45.7 | 36.7 | 44.5 | 41.9 |
| Height (inches) | 68.4 | 71.75 | 69.9 | 65.7 | 67.5 | 66.9 |
| Weight (lbs) | 184.2 | 192.5 | 187.9 | 216.7 | 175.7 | 189.3 |
| BMI | 27.9 | 26.5 | 27.3 | 34.8 | 27.2 | 29.7 |
| Pain Duration (months) | 40.6 | 19 | 31 | 82.0 | 20.8 | 41.2 |
Key: NON: non-insertional tendinopathy; INS: insertional tendinopathy.
Table 2.
Group 1 (MT +Ex) and Group 2 (TDN + MT + Ex) mean scores for tests and measures, change scores from baseline to 4 weeks and baseline to 3 months.
| Tests and Measures | Baseline | 4 week | 3 month | Mean within group change (Baseline to 4 weeks) | Mean within group change (Baseline to 3 months) | p-value |
|---|---|---|---|---|---|---|
| Group 1 | ||||||
| FAAM (%) | 59 | 86 | 85 | 24 | 28 | *0.002 |
| NPRS | 5.8 | 2.7 | 1.7 | 3.1 | 4 | *0.001 |
| GROC | NA | 4.8 | 6.1 | NA | 1.3 | *0.046 |
| PPT (kg) | 2.3 | 4.7 | 5.3 | 2.4 | 3.3 | *0.000 |
| Strength (reps) | 17.2 | 24.5 | 26.2 | 7.3 | 9 | *0.008 |
| Group 2 | ||||||
| FAAM (%) | 59 | 78 | 78 | 19 | 19 | *0.006 |
| NPRS | 5.2 | 3.3 | 3.2 | 1.9 | 2.0 | *0.018 |
| GROC | NA | 5.3 | 5.3 | NA | 0 | 1 |
| PPT (kg) | 2.5 | 4 | 4.8 | 1.8 | 2.3 | *0.006 |
| Strength (reps) | 9.4 | 15.3 | 17.6 | 5.9 | 8.2 | *0.007 |
* P < .05
Table 3.
Between group analysis.
| Variable | Sig |
|---|---|
| FAAM at 4 weeks | 0.388 |
| FAAM at 3 months | 0.834 |
| NPRS at 4 weeks | 0.548 |
| NPRS at 3 months | 0.1 |
| GROC at 4 weeks | 0.063 |
| GROC at 3 months | 0.348 |
| PPT at 4 weeks | 0.257 |
| PPT at 3 months | 0.526 |
| Strength at 4 weeks | 0.916 |
| Strength at 3 months | 0.673 |
A between group analysis was performed with Mann Whitney U. There were no statistically significant differences between groups for any of the dependent variables (Table 3).
Change scores for baseline to discharge at 4 weeks and baseline to 3-month follow-up were compared to the MCID for the FAAM, NPRS and GROC. The change score means for the FAAM between the baseline to the discharge at 4 weeks as well as the baseline to 3-month follow-up exceeded the MCID of 8 [43] for both data collection points in both groups. The individual subject results for the FAAM are reported in Figures 2 and 3. The MCID for the NPRS is 2 [46]. The change score means for the NPRS between the baseline to the discharge at 4 weeks as well as the baseline to 3-month follow-up exceeded the MCID for MT+Ex group. The TDN+MT+Ex group means for change exceeded the MCID for only the baseline to 3-month follow-up. The NPRS significantly improved for both groups. The MCID for the GROC is +4 [45]. The means for both groups exceeded the MCID at both the 4-week and 3-month measures in both groups.
Comparison of results between participants with insertional and non-insertional tendinopathy within each group is found in Table 4. Results at the 4-week and 3-month data collection reveal similar outcomes for each variable.
Table 4.
Insertional vs Non-insertional Comparison.
| Variable | Group | IE | DC | 3 month |
|---|---|---|---|---|
| FAAM | 1 -NON | 66% | 84% | 85% |
| 1 – INS | 49% | 82% | 84% | |
| 2 – Non | 62% | 78% | 79% | |
| 2- INS | 50% | 73% | 77% | |
| NPRS | 1 -NON | 5.5 | 2.7 | 2.3 |
| 1 – INS | 6.3 | 2.8 | 0.8 | |
| 2 – Non | 4.9 | 3.1 | 2.9 | |
| 2- INS | 6 | 3.7 | 4 | |
| GROC | 1 -NON | 1.4 | 1.6 | |
| 1 – INS | 1.1 | 1.9 | ||
| 2 – Non | 1.4 | 1.6 | ||
| 2- INS | 1.7 | 1.3 | ||
| PPT | 1 -NON | 1.2 | 2.5 | 2.3 |
| 1 – INS | 1 | 2.1 | 2.9 | |
| 2 – Non | 1.4 | 2.1 | 2.3 | |
| 2- INS | 1 | 2.3 | 2.7 | |
| Strength | 1 -NON | 1.5 | 2.2 | 2.3 |
| 1 – INS | 1.1 | 2.1 | 2.5 | |
| 2 – Non | 1.4 | 2.1 | 2.3 | |
| 2- INS | 1.5 | 2.2 | 2.3 |
Key: Group 1: MT+Ex; Group 2: TDN+MT+Ex; NON: non-insertional tendinopathy; INS: insertional tendinopathy.
The effect size was calculated for groups 1 and 2 by using Kendall’s Coefficient of Concordance. Kendall’s W can be used for effect size calculations in SPSS [49]. The effect size was 0.663 for the MT + Ex group and .416 for the TDN+MT+Ex group.
Discussion
The primary aim of this study was to test the feasibility of a randomized controlled trial investigating the effects of TDN to myofascial trigger points, manual therapy and eccentric exercise compared to manual therapy and eccentric exercise alone for individuals with Achilles tendinopathy. Feasibility is determined by meeting the primary and secondary objectives.
Analysis of the primary objectives pertaining to subject recruitment is concerning. Primary objective #1 was not met. Subject recruitment for this study was slow with a total of 34 subjects eligible to participate in a 3-year time span. The initial study inclusion criteria were limited to mid-portion Achilles tendinopathy but were revised after the 1st year to include insertional tendinopathy to improve subject recruitment. Recruitment did increase in the second year of data collection but then dropped over the next year for a total of 22 subjects entering the study.
There were two key factors that were attributed to the low recruitment: rising out-of-pocket costs and changes in referring provider health system networks. Health insurance deductibles and co-pays for physical therapy services increased significantly in the first year of data collection in the regions of data collection. Additionally, several data collection sites had referring providers for Achilles tendinopathy changing health-care networks leaving these clinics out of network and thus a higher cost for care. Communication from referring providers indicated that their patients were not willing to pay the higher cost for treatment at clinics out of network. However, these data were not collected.
Additional factors contributing to low recruitment were related to the barriers to participation. A total of 12 subjects were eligible but declined participation. This amounts to 17.1% of the total subjects evaluated for the study (n = 70) but 35.3% of the subjects eligible to participate (n = 34). Barriers to their participation included aversion to needle penetration to painful trigger points, financial constraints and limited time available to complete the treatment protocol. In this study, 4 of the 70 patients (5%) eligible to participate declined due to an aversion of needles.
Primary objective #3 was related to the practicality of enrolling subjects during normal clinic operations. All physical therapists reported average treatment volumes over 14 patients per 8-h day. They rated the protocol to be manageable and were able to maintain their typical patient schedule. Therefore, this protocol could be used in similar clinical practices for a future study.
The secondary objectives of this study were related to the clinical outcomes of the study.
Secondary objective #1 was met as the 4 week, end of treatment attrition rate was 18.1%. There were four subjects that dropped out of the study. Two subjects dropped out due to a family emergency and time commitment. There were two subjects who dropped out due to poor progress with treatment, one from each group, resulting in a 9.1% drop out rate due to poor progress.
Secondary objective #2 was also met. There were no serious adverse events reported in this study. Post-treatment soreness was commonly reported from subjects in both groups. Based on the criteria from Brady et al., soreness from TDN is considered a minor adverse event and was commonly reported in their study [50]. Post-exercise soreness is also a common report with eccentric training for patients with Achilles tendinopathy and may have contributed to the post-treatment soreness [11].
Secondary objective #3 was not met. The attrition rate for the 3-month follow-up for this study was poor at 68% when factoring in dropouts and is a major limitation for this study. Factors that may have attributed to this include the requirement of subjects to return to the participating clinic. There was an incentive for the subjects to attend each treatment session as they were receiving treatment that was effective in reducing their pain and improving their tolerance for functional activities. However, once they were discharged from treatment there was no further incentive to return aside from their obligation to the study.
Secondary objective #4 pertained to reporting treatment effects. The within group analysis resulted in statistically significant improvements for pain reduction, strength and functional outcomes for each group. However, there was no significant difference between groups for any dependent variables. The results of the within and between group analysis may be misleading due to the low recruitment and low power for the 4-week analysis as well as the poor follow-up response rate for the 3-month analysis.
The results of this study indicate a large randomized trial with the present treatment protocol may not be feasible due to the low subject recruitment combined with the barriers to participation and out-of-pocket costs for physical therapy management. A large number of investigators would be needed to recruit subjects to meet the power analysis in an acceptable 1–2 year data collection period. These issues may be surmountable if the study can be conducted within a large health system with a wide referral network, a closed system such as the United States Department of Defense or in a country with a national health system where there may be greater access to eligible participants where out-of-pocket costs may not be problematic.
In the event that this study would be replicated, recommendations for modifications to this study design include:
It is recommended the study would be conducted in a closed system such as the Department of Defense or country with a national health insurance.
The study treatment protocol should be pragmatic to allow the data collection to be consistent with real-life conditions in clinical practice.
Treatment frequency and duration should not be standardized. This may enable subjects with financial constraints to participate in the study.
A financial incentive may increase subject recruitment and reduce the attrition rate for follow-up data collection.
Data collection for long-term follow-up should allow the option for subjects to complete self-report measures via video conference to verify identity. This would also allow the researcher to view the subject performing the heel raise exercise and record these data. This does eliminate the PPT variable from the follow-up data collection. Normative data have not been published for PPT to the Achilles tendon, nor has data correlating PPT measures to functional activity status. Therefore, the value of PPT to the results of future studies may need to be weighed against the importance of reducing the attrition rate by collecting follow-up data.
Conclusion
In conclusion, the results of this study indicate a large randomized trial with the present treatment protocol is not feasible due to low subject recruitment and retention. This study could be conducted in a region or health system with access to a larger patient population or where financial constraints may be overcome. The study design and treatment protocol could be modified for large RCT to investigate the effect of TDN on Achilles tendinopathy. Two of the three conditions for the feasibility of the treatment protocol were met. However, the issues surrounding recruitment and attrition overshadow these conditions. A within group analysis indicates a significant improvement that was also clinically meaningful for pain, strength and functional outcomes in both groups. There was a large attrition for the 3-month follow-up data which increases the probability for a type II error [34]. Follow-up studies are needed to determine the effect of TDN for Achilles tendinopathy and to compare the results of the intervention to other treatment options available to people with this diagnosis.
Acknowledgments
The authors would like to acknowledge the following physical therapists for their contributions to this study: Matt Ware, Adam Autry, John Autry, Matt Tuttle, Blake Allen, Taylor Comford, Susan Daugherty, Kelli Brummer and Beth Dessner. The additional time and energy they devoted to this study is truly appreciated.
Biographies
Dr. A. Koszalinski is the assistant Dean of the South College DPT program. He is board certified in Orthopedic Physical Therapy and a Fellow of the American Academy of Orthopedic Manual Physical Therapists. He has presented at multiple physical therapy conferences and seminars. His research interests include manual therapy, trigger point dry needling and best practices for instruction in DPT programs.
Dr. T. Flynn is board certified in Orthopaedic Physical Therapy (OCS), a Fellow of the American Academy of Orthopaedic Manual Physical Therapists (FAAOMPT), a Fellow of the American Physical Therapy Association (FAPTA) and a frequent presenter at state, national, and international meetings. Dr. Flynn is widely published with over 85 peer-reviewed manuscripts on musculoskeletal disorders and chronic spinal pain. He has received numerous professional awards. Dr. Flynn is a past President of the American Academy of Orthopaedic Manual Physical Therapists. He is a Professor of Physical Therapy at South College, TN. Dr. Flynn is a world-renowned clinician dedicated to providing the highest quality care possible at Colorado In Motion in Fort Collins, CO. His primary clientele includes clients suffering from back pain, chronic spinal pain, failed back surgeries, and chronic pain disorders. He is passionate about ensuring that clients can and should expect better healthcare and the ability to have fitness and vitality throughout their life. He can be heard weekly on the Pain Reframed Podcast.
Dr. M. Hellman was associate professor and Chair of Physical Therapy Department at Nova Southeastern University. She is widely published and has presented at numerous conferences.
Dr. J. A. Cleland is actively involved in numerous clinical research studies investigating the effectiveness of manual physical therapy and exercise in the management of spine and extremities disorders. He has published over 250 manuscripts in peer–reviewed journals. He is an Editor for the Journal of Orthopaedic and Sports Physical Therapy. He is currently an author/editor on 4 text books. Dr Cleland is a well–known speaker at both the national and international level.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Egger AC, Berkowitz MJ.. Achilles tendon injuries. Curr Rev Musculoskelet Med. 2017. Mar;10(1):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Carcia CR, Martin RL, Houck J, et al. Achilles pain, stiffness, and muscle power deficits: Achilles tendinitis. J Orthop Sports Phys Ther. 2010;40(9):A1–26. [DOI] [PubMed] [Google Scholar]
- [3].Academy A, Board OS, December D. The Diagnosis and Treatment of Acute Achilles Tendon Rupture Adopted by the American Academy of Orthopaedic Surgeons Board of Directors. 2009.
- [4].Sweeting K Conservative care for mid-portion Achilles tendinopathy | Podiatry Today. Cited 2014 Jul 15. Available from: http://www.podiatrytoday.com/conservative-care-mid-portion-Achilles-tendinopathy.
- [5].Wezenbeek E, De Clercq D, Mahieu N, et al. Activity-induced increase in Achilles tendon blood flow is age and sex dependent. Am J Sports Med. 2018. Sep;46(11):2678–2686. [DOI] [PubMed] [Google Scholar]
- [6].Schepsis AA, Jones H, Haas AL, Achilles tendon disorders in athletes. Am J Sports Med. 30(2):287–305. Cited 2013 Aug 5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11912103. [DOI] [PubMed] [Google Scholar]
- [7].Johannsen FE, Gam AN.. Achillodynia is not just a sports injury. Ugeskr Laeger. 2010;172(48):3325–3329. [PubMed] [Google Scholar]
- [8].Rolf C, Movin T. Etiology, histopathology, and outcome of surgery in achillodynia. Foot Ankle Int. 1997;18(9):565–569. [DOI] [PubMed] [Google Scholar]
- [9].Lysholm J, Wiklander J, Injuries in runners. Am J Sports Med. 15(2):168–171. Cited 2013 Aug 5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3578639. [DOI] [PubMed] [Google Scholar]
- [10].Ohberg L. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up * Commentary. Br J Sports Med. 2004;38(1):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rees JD, Wolman RL, Wilson A. Eccentric exercises; why do they work, what are the problems and how can we improve them? Br J Sports Med. 2009;43(4):242–246. [DOI] [PubMed] [Google Scholar]
- [12].Martin RL, Chimenti R, Cuddeford T, et al. Achilles pain, stiffness, and muscle power deficits: midportion Achilles tendinopathy revision 2018. J Orthop Sports Phys Ther. 2018. May;48(5):A1–A38. [DOI] [PubMed] [Google Scholar]
- [13].Silbernagel KG, Thomeé R, Eriksson BI, et al. Full symptomatic recovery does not ensure full recovery of muscle-tendon function in patients with Achilles tendinopathy. Br J Sports Med. 2007;41(4):276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Willits K, Amendola A, Bryant D, et al. Operative versus nonoperative treatment of acute Achilles tendon ruptures: a multicenter randomized trial using accelerated functional rehabilitation. J Bone Joint Surg Am. 2010;92(17):2767–2775. [DOI] [PubMed] [Google Scholar]
- [15].Silbernagel KG, Brorsson A, Lundberg M. The majority of patients with Achilles tendinopathy recover fully when treated with exercise alone: a 5-year follow-up. Am J Sports Med. 2011;39(3):607–613. [DOI] [PubMed] [Google Scholar]
- [16].Gärdin A, Movin T, Svensson L, et al. The long-term clinical and MRI results following eccentric calf muscle training in chronic Achilles tendinosis. Skeletal Radiol. 2010. May;39(5):435–442. [DOI] [PubMed] [Google Scholar]
- [17].de Vos RJ, Weir A, Tol JL, et al. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011. Apr;45(5):387–392. [DOI] [PubMed] [Google Scholar]
- [18].Rompe JD, Furia J, Maffulli N. Eccentric loading compared with shock wave treatment for chronic insertional Achilles tendinopathy. A randomized, controlled trial. J Bone Joint Surg Am. 2008. Jan;90(1):52–61. [DOI] [PubMed] [Google Scholar]
- [19].Luan S, Zhu ZM, Ruan JL, et al. Randomized trial on comparison of the efficacy of extracorporeal shock wave therapy and dry needling in myofascial trigger points. Am J Phys Med Rehabil. 2019. Aug;98(8):677–684. [DOI] [PubMed] [Google Scholar]
- [20].Simons DG, Travell JGSL. Travell and Simons’ myofascial pain and dysfunction: the trigger point manual. vol 1: upper half of body. 2nd ed. Baltimore MD: Williams & Wilkins; 1999. [Google Scholar]
- [21].Renan-Ordine R, Alburquerque-Sendín F, de Souza DPR, et al. Effectiveness of myofascial trigger point manual therapy combined with a self-stretching protocol for the management of plantar heel pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2011;41(2):43–50. [DOI] [PubMed] [Google Scholar]
- [22].Ong J, Claydon LS. The effect of dry needling for myofascial trigger points in the neck and shoulders: a systematic review and meta-analysis. J Bodyw Mov Ther. 2014;18(3):390–398. [DOI] [PubMed] [Google Scholar]
- [23].Mejuto-Vázquez MJ, Salom-Moreno J, Ortega-Santiago R, et al. Short-term changes in neck pain, widespread pressure pain sensitivity, and cervical range of motion after the application of trigger point dry needling in patients with acute mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2014;44(4):252–260. [DOI] [PubMed] [Google Scholar]
- [24].Cotchett MP, Landorf KB, Munteanu SE. Effectiveness of dry needling and injections of myofascial trigger points associated with plantar heel pain: a systematic review. J Foot Ankle Res. 2010;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lagerquist A, So B, Roos EM, et al. Clinical improvement after 6 weeks of eccentric. Scand J Med Sci sports. 2004;286–295. DOI: 10.1111/j.1600-0838.2004.00378.x. [DOI] [PubMed] [Google Scholar]
- [26].Espejo-Antúnez L, Tejeda JF, Albornoz-Cabello M, et al. Dry needling in the management of myofascial trigger points: a systematic review of randomized controlled trials. Complement Ther Med. 2017. Aug;33:46–57. Epub 2017 Jun 15. [DOI] [PubMed] [Google Scholar]
- [27].Lucas N, Macaskill P, Irwig L, et al. Reliability of physical examination for diagnosis of myofascial trigger points: a systematic review of the literature. Clin J Pain. 2009. Jan;25(1):80–89. [DOI] [PubMed] [Google Scholar]
- [28].Kearns G, Fernández-De-Las-Peñas C, Brismée JM, et al. New perspectives on dry needling following a medical model: are we screening our patients sufficiently? J Man Manip Ther. 2019. Jul;27(3):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Perreault T, Dunning J, Butts R. The local twitch response during trigger point dry needling: is it necessary for successful outcomes? J Bodyw Mov Ther. 2017. Oct;21(4):940–947. [DOI] [PubMed] [Google Scholar]
- [30].Griswold D, Wilhelm M, Donaldson M, et al. The effectiveness of superficial versus deep dry needling or acupuncture for reducing pain and disability in individuals with spine-related painful conditions: a systematic review with meta-analysis. J Man Manip Ther. 2019. Jul;27(3):128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Amadeo K The rising cost of health care by year and its causes. The Balance. 2018. Sept 15. Cited 2018 Oct 27. Available from: https://www.thebalance.com/causes-of-rising-healthcare-costs-4064878.
- [32].Abutaleb Y U.S. healthcare spending to climb 5.3 percent in 2018: agency. Reuters. 2018. Feb 14. Cited 2018 Oct 20. Available from: https://www.reuters.com/article/us-usa-healthcare-spending/u-s-healthcare-spending-to-climb-5-3-percent-in-2018-agency-idUSKCN1FY2ZD.
- [33].Kaiser Family Foundation . Employer health benefits: 2018 summary of findings. 2018. Oct 03. Cited 2018 Oct 20. Available from: https://www.kff.org/health-costs/report/2018-employer-health-benefits-survey/.
- [34].Carvalho E, Bettger JP, Goode AP. Insurance coverage, costs, and barriers to care for outpatient musculoskeletal therapy and rehabilitation services. NCMJ. 2017;78(5):312–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].APTA . Fair physical therapy copays.2018. Jun 26. Cited 2018 Oct 20. Available from: http://www.apta.org/StateIssues/FairCopays/.
- [36].Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract. 1995. Aug;41(2):169–175. [PubMed] [Google Scholar]
- [37].Tough EA, White AR, Richards SH, et al. Myofascial trigger point needling for whiplash associated pain–a feasibility study. Man Ther. 2010. Dec;15(6):529–535. [DOI] [PubMed] [Google Scholar]
- [38].Abbott JH. The distinction between randomized clinical trials (RCTs) and preliminary feasibility and pilot studies: what they are and are not. J Orthop Sports Phys Ther. 2014. Aug;44(8):555–558. [DOI] [PubMed] [Google Scholar]
- [39].Orsmond GI, Cohn ES. The distinctive features of a feasibility study: objectives and guiding questions. OTJR (Thorofare N J). 2015. Jul;35(3):169–177. [DOI] [PubMed] [Google Scholar]
- [40].Eldridge SM, Chan CL, Campbell MJ, et al. PAFS consensus group. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016. Oct 21;2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dommerholt J, Fernandez-de-las-Penas C, eds. Trigger point dry needling: an evidence and clinical-based approach. Oxford: Churchill Livingstone Elsevier; 2013. [Google Scholar]
- [42].Chen Q, Bensamoun S, Basford JR, et al. Identification and quantification of myofascial taut bands with magnetic resonance elastography. Arch Phys Med Rehabil. 2007;88(12):1658–1661. [DOI] [PubMed] [Google Scholar]
- [43].Martin RL, Irrgang JJ, Burdett RG, et al. Evidence of validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005;26(11):968–983. [DOI] [PubMed] [Google Scholar]
- [44].Wagner Force Measurement Instruments . Cited 2014 Oct 13. Available from: http://www.paintest.com/fpk_dial_pain_tester_algometer.php.
- [45].Jaeschke R, Singer J, Guyatt G. Ascertaining the minimally clinically important difference. Control Clin Trials. 1989;10(4):407–415. [DOI] [PubMed] [Google Scholar]
- [46].Farrar JT, Young JP Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. [DOI] [PubMed] [Google Scholar]
- [47].Maffulli N, Kenward MG, Testa V, et al. Clinical diagnosis of Achilles tendinopathy with tendinosis. Clin J Sport Med. 2003. Jan;13(1):11–15. [DOI] [PubMed] [Google Scholar]
- [48].Sealed Envelope . Create a randomization list. Cited 2014 Nov 17. Available from: https://www.sealedenvelope.com/simple-randomiser/v1/lists
- [49].Tomczak MJ, Tomczak E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014;1(21):19–25. [Google Scholar]
- [50].Brady S, McEvoy J, Dommerholt J, et al. Adverse events following trigger point dry needling: a prospective survey of chartered physiotherapists. J Man Manip Ther. 2014;23(3):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Sweeting K Conservative care for mid-portion Achilles tendinopathy | Podiatry Today. Cited 2014 Jul 15. Available from: http://www.podiatrytoday.com/conservative-care-mid-portion-Achilles-tendinopathy.


