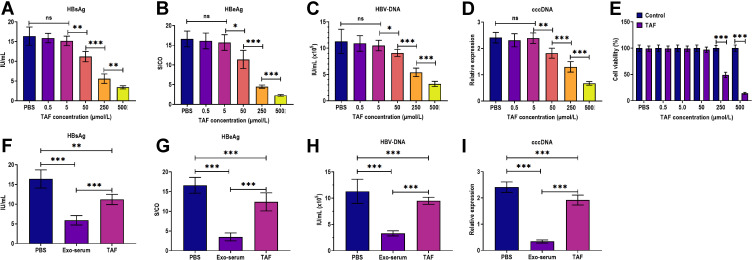
Figure 3.
The antiviral effects of exosomes derived from the serum of CHB patients and TAF treatment. (A) The HBsAg level in the culture supernatants of HepAD38 cells treated with 0.5, 5, 50, 250, and 500 µmol/L TAF for 48 h, respectively. (B) The HBeAg level in the culture supernatants of HepAD38 cells treated with 0.5, 5, 50, 250, and 500 µmol/L TAF for 48 h, respectively. (C) The HBV DNA level in the culture supernatants of HepAD38 cells treated with 0.5, 5, 50, 250, and 500 µmol/L TAF for 48 h, respectively. (D) The intracellular HBV cccDNA level of HepAD38 cells treated with 0.5, 5, 50, 250, and 500 µmol/L TAF for 48 h, respectively. (E) Cell viability of HepAD38 cells treated with 0.5, 5, 50, 250, and 500 µmol/L TAF for 48 h, respectively, as detected by CCK-8 assay. (F) The HBsAg level in the culture supernatants of HepAD38 cells treated with exosomes derived from the serum of CHB patients at 48 weeks after receiving daily TAF treatment (25 mg) (Exo-serum) (10 μg/mL) or TAF treatment (50 µmol/L) for 48 h. (G) The HBeAg level in the culture supernatants of HepAD38 cells treated with Exo-serum (10 μg/mL) or TAF treatment (50 µmol/L) for 48 h. (H) The HBV DNA level in the culture supernatants of HepAD38 cells treated with Exo-serum (10 μg/mL) or TAF treatment (50 µmol/L) for 48 h. (I) The intracellular HBV cccDNA level of HepAD38 cells treated with Exo-serum (10 μg/mL) or TAF treatment (50 µmol/L) for 48 h. *p < 0.05, **p < 0.01, ***p < 0.001.