Figure 1.
Crystal structures of MINDY1 in complex with K48-linked di-ubiquitin
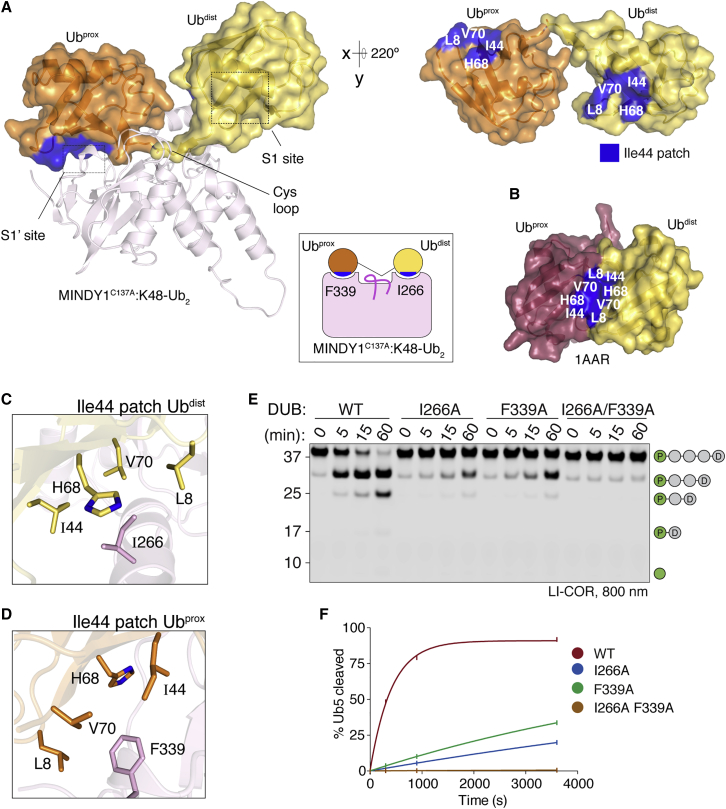
(A) The MINDY1C137A:K48-Ub2 complex crystal structure is shown with MINDY1 in illustration (light pink). Ub molecules are depicted with transparent surfaces (tv-orange:Ubprox and yelloworange:Ubdist). I44 patches on Ub are colored blue, and an alternate view of the bound diUb rotated by 220° along the x axis is shown on the right side. Schematic representation of MINDY1C137A:K48-Ub2 complex (inset).
(B) Surface representation of the closed conformation of K48-Ub2 (PDB: 1AAR) with I44 patches highlighted in blue.
(C and D) Close-up views of the key residues on the MINDY1 S1 and S1′ sites and their interactions with the I44 patches on Ubdist and Ubprox.
(E) DUB assay monitoring cleavage of K48-linked pentaUb, in which Ubprox is fluorescently labeled by MINDY1 and indicated mutants.
(F) Quantification of pentaUb hydrolysis shown in (D). The percentage of the total intensities of Ub4, Ub3, Ub2, and Ub1 formed is shown on the y axis. n = 2; mean ± SD.
See also Figures S1 and S2.