Figure 2.
Cys loop mobility regulates DUB activity
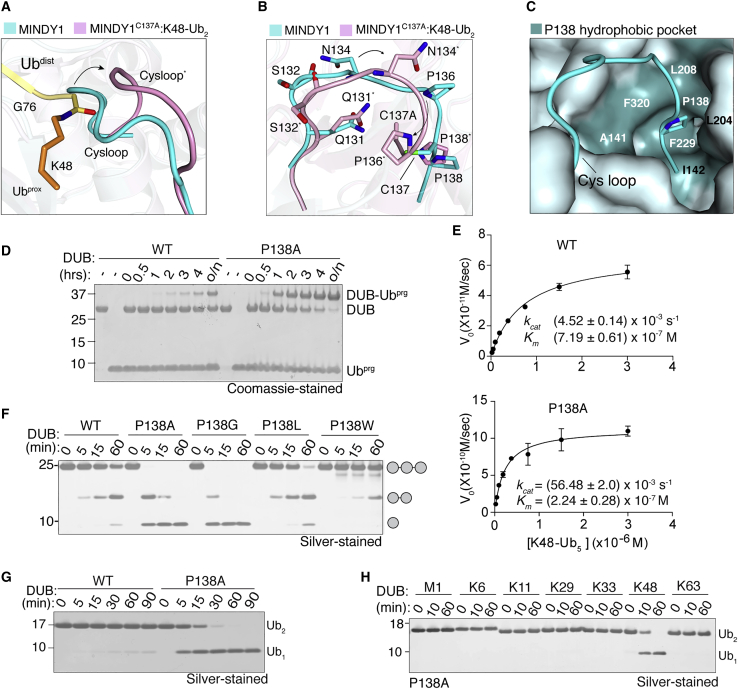
(A) Representation of the Cys loop in a superposition of MINDY1apo (cyan) and MINDY1C137A:K48-Ub2 complex (pink). The isopeptide bond between K48 of Ubprox (orange) and G76 of Ubdist (yellow) is shown in sticks.
(B) Close-up view of (A) showing amino acid side chain rearrangements (side view).
(C) Surface representation of the hydrophobic pocket in MINDY1apo that accommodates the Cys loop residue P138.
(D) Coomassie-stained gel comparing activity of MINDY1 WT and P138A to UbPrg in a time course.
(E) Steady-state kinetics of K48-linked pentaUb cleavage by MINDY1 WT and P138A mutant derived from reactions with varying concentrations of fluorescently labeled Ub5 (n = 2; means ± SDs).
(F) Silver-stained gel comparing cleavage of K48-Ub3 by MINDY1 WT and indicated mutants.
(G) DUB assay comparing cleavage of K48-Ub2 by MINDY1 WT and P138A mutants.
(H) DUB assay monitoring cleavage of diUb of 7 different linkage types by MINDY1 P138A.
See also Figure S3.