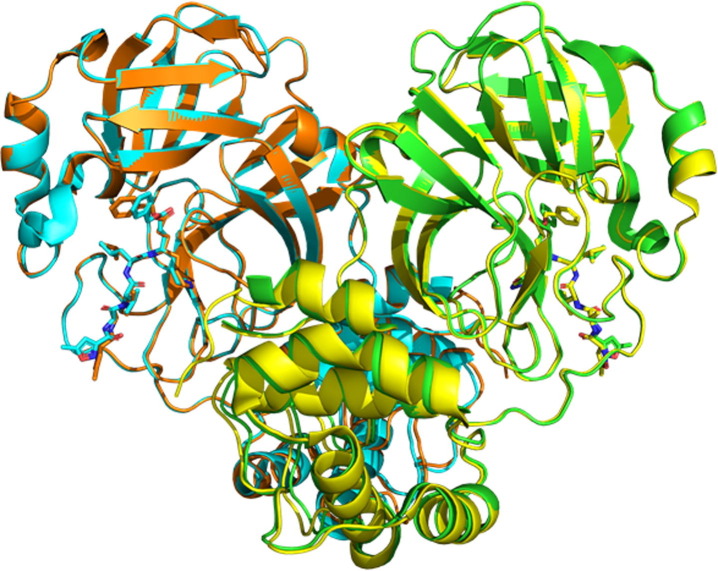
Figure 1.
Illustrations of SARS-CoV-2 3CLpro (PDB:7bqy, Chain A (green) and Chain B (cyan)) and SARS-CoV 3CLpro (PDB:2hob, Chain A (yellow) and Chain B (orange)), superimposed at the core region of the chymotrypsin fold. Both have homodimeric structures of C2 symmetry complexed with N3 (stick; a peptide-mimic compound), which are superimposable (Cα RMSD = 0.68 Å), although the terminal moieties of N3 have some deviation. The upper domain is the chymotrypsin fold named domains I and II, whereas the lower helical domain is domain III.