Abstract
Objective
We sought to evaluate the impact on survival of tumor burden and surgical complexity in relation to the number of cycles of neoadjuvant chemotherapy (NACT) in patients with advanced ovarian cancer (OC) with minimal (CC-1) or no residual disease (CC-0).
Methods
This retrospective study included patients with International Federation of Gynaecology and Obstetrics IIIC–IV stage OC who underwent debulking surgery at 4 high-volume institutions between January 2008 and December 2015. We assessed the overall survival (OS) of primary debulking surgery (PDS group), early interval debulking surgery after 3–4 cycles of NACT (early IDS group) and delayed debulking surgery after 6 cycles (DDS group) with CC-0 or CC-1 according to peritoneal cancer index (PCI) and Aletti score.
Results
Five hundred forty-nine women were included: 175 (31.9%) had PDS, 224 (40.8%) early IDS and 150 (27.3%) DDS. Regardless of Aletti score, median OS after PDS was significantly higher than after early IDS or DDS, but the survival difference was higher in women with an Aletti score <8. Among patients with PCI ≤10, median OS after PDS was significantly higher than after early IDS or DDS. In women with PCI >10, there were no differences between PDS and early IDS, but DDS was associated with decreased OS.
Conclusion
The benefit of complete PDS compared with NACT was maximal in patients with a low complexity score. In patients with low tumor burden, there was a survival benefit of PDS over early IDS or DDS. In women with high tumor load, DDS impaired the oncological outcome.
Keywords: Ovarian Neoplasms, Fallopian Tube Neoplasms, Peritoneal Neoplasms, Cytoreduction Surgical Procedures, Neoadjuvant Therapy, Tumor Burden
INTRODUCTION
Complete cytoreduction with no residual disease is the main prognostic factor in advanced epithelial ovarian cancer (OC) [1,2]. The gold standard treatment in these patients is the combination of complete cytoreductive surgery (CRS) with platinum and taxane-based chemotherapy [3]. In patients with completely resectable disease and good performance status, primary debulking surgery (PDS) is the first option to consider, as it has been consistently associated with improved survival outcomes in retrospective studies [4,5,6]. Neoadjuvant chemotherapy (NACT) is associated with lower morbidity and postoperative mortality [7,8], and it is preferred in medically non-operable patients or in the low likelihood of achieving complete cytoreduction, with non-inferior survival benefit [7,9,10]. Classically, interval debulking surgery (IDS) is performed after three or four cycles of NACT, and 2 or 3 cycles of adjuvant chemotherapy are delivered after CRS to complete a total of 6 cycles of chemotherapy. However, reports evaluating the role of IDS after more than four cycles of NACT are controversial. While some have shown that survival is similar to that of patients undergoing IDS after three cycles of NACT [11,12,13,14], other studies have described an impaired prognosis of delayed IDS [15,16].
The incorporation of extensive upper abdominal procedures in CRS has increased the rate of optimal cytoreduction as well as improved the survival of advanced OC patients [17]. However, ultraradical procedures are associated with higher morbidity and postoperative mortality [18,19,20]. Likewise, high intraabdominal tumor load has a negative impact on survival [21,22,23]. The aim of our study was to evaluate the survival impact of tumor burden and surgical complexity in relation to the number of NACT cycles in patients with advanced OC with minimal or no residual disease after CRS.
MATERIALS AND METHODS
1. Patients and study design
A computer-generated search in the institutional patient database was performed to identify retrospectively all patients who underwent upfront, interval or closure complete CRS with complete cytoreduction (CC-0) or cytoreduction to minimal residual disease (CC-1) for stage IIIC–IV epithelial ovarian, fallopian, or primary peritoneal cancer between January 2008 and December 2015 in four institutions meeting the requirements of the European Society of Gynaecological Oncology quality indicators from France and Spain. National and Institutional Review Board approval was obtained from our centres (SLN/MFI/AR193997 and HULP code PI-3432).
2. Preoperative assessment, surgery principles and chemotherapy treatment
All patients underwent a preoperative imaging study including a computed tomography (CT) of the chest, abdomen and pelvis. In selected cases of extra-abdominal disease suspicion, a positron emission tomography (PET)/CT was performed. An exploratory laparoscopy was routinely performed at diagnosis to assess resectability and to obtain a histological diagnosis.
All surgical procedures were performed or supervised by experienced oncological surgeons. The surgical technique of CRS was performed following Surgarbaker principles of peritonectomy [24]. The extent and distribution of the disease throughout the 13 abdominopelvic regions were evaluated with the peritoneal cancer index (PCI) [25]. The surgical goal was to achieve complete cytoreduction, evaluated using the Completeness of Cytoreduction score (CC-0: no residual tumor; CC-1: residual disease less than 2.5 mm in diameter; CC-2: residual nodules between 2.5 mm and 2.5 cm; and CC-3 residual nodules greater than 2.5 cm or a confluence of unresectable disease) [25]. Hysterectomy and bilateral salpingo-oophorectomy, infragastric omentectomy and pelvic plus paraaortic lymphadenectomy were the procedures systematically performed during CRS. However, some patients referred from external hospitals had already undergone uni- or bilateral salpingo-oophorectomy with or without hysterectomy at diagnosis. Moreover, as the study period preceded the LION trial [26], lymphadenectomy could be spared only in some selected patients without lymph node involvement at diagnosis to decrease operative time and surgical morbidity. The Aletti score was used to quantify surgical complexity with a cut-off value ≥8 being considered as high complexity [27]. Postoperative complications were documented according to the Clavien-Dindo classification [28].
Patients with deep infiltration of the small bowel mesentery, diffuse carcinomatosis involving large parts of the small bowel, stomach, infiltration of the duodenum or pancreas (not limited to the pancreatic tail) were considered non-resectable and were selected for primary chemotherapy. NACT was also indicated in patients unfit to withstand multivisceral resection due to medical co-morbidities or poor performance status, or when too extensive surgery was needed to achieve complete cytoreduction. After three or four cycles of platinum and taxane-based chemotherapy, a clinical, biological and imaging evaluation by thoraco-abdomino-pelvic CT or PET/CT was performed. In the event of poor response or bad performance status, three additional cycles of NACT were administered before IDS. In selected patients with stable disease on CT after NACT, an exploratory laparoscopy was performed before IDS to assess resectability.
Adjuvant chemotherapy was delivered, when feasible, within one or two months after CRS with carboplatin and paclitaxel until a total of six cycles had been completed. In the event of high tumor burden, CC-1 or poor response to NACT, antiangiogenic maintenance treatment with bevacizumab was added after discussion by the tumor board. When surgery was performed after 6 cycles of NACT, two to three additional cycles of chemotherapy were added to the antiangiogenic maintenance treatment with bevacizumab. No maintenance treatment with poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors was administered during the study period.
Patients with residual disease ≥2.5 mm (≥CC-2) and patients with non-epithelial subtype histology or borderline tumors were excluded from the study.
3. Study data
Medical databases were carefully examined to collect all relevant information. Patient demographic data, World Health Organization (WHO) performance status, cancer antigen-125 (CA-125) dosage, ascites at diagnosis, NACT, PCI scores recorded during CRS, surgical procedures, surgical complexity according to Aletti score, histologic data, adjuvant treatment and follow-up data were retrieved from medical records.
Patients were classified in 3 groups according to the surgical timing: upfront surgery and 6 cycles of adjuvant chemotherapy (PDS group), IDS after 3–4 cycles of NACT and 2–3 cycles of adjuvant chemotherapy to achieve a total of 6 cycles (early IDS group), and delayed debulking surgery (DDS) after 6 cycles of NACT (DDS group). The latter group included patients undergoing delayed IDS after 6 cycles of NACT and receiving 2 additional cycles of adjuvant chemotherapy due to poor response to NACT, and patients undergoing closure debulking surgery after 6 cycles of NACT without adjuvant chemotherapy.
4. Statistical analysis
Data were summarised by frequency and percentage for categorical variables and by median and range for continuous variables. Comparisons between groups were performed using the χ2 or Fisher's exact test for categorical variables and the Kruskal-Wallis test for continuous variables. Overall survival (OS) was defined as the time from diagnosis until death from any cause or last follow-up news (censored data) and was estimated using the Kaplan-Meier method. Comparisons between groups were performed using the Logrank test. The Cox proportional hazards model was used to perform multivariable analysis and to estimate hazard ratio (HR) and adjusted hazard ratios (HRadj) with their 95% confidence interval (95% CI). All statistical tests were two-sided and p-values <0.05 were considered statistically significant. Statistical analyses were conducted using STATA 16 (StataCorp, College Station, TX, USA) software.
RESULTS
During the study period, 549 patients met the inclusion criteria (Fig. S1). Among them, 175 patients (31.9%) had upfront surgery, 224 (40.8%) underwent early IDS, and 150 (27.3%) had DDS. Within patients undergoing DDS, 106 had a delayed IDS and 44 underwent closure debulking surgery. Baseline characteristics of the three subgroups are shown in Table 1. Patients in the PDS group were significantly younger than those in the early IDS and DDS groups and they had a significantly better performance status. CA-125 was significantly higher with the increasing number of cycles of NACT. In the PDS group, there was a higher proportion of patients with stage IIIC disease and non-serous histology, and ascites at diagnosis was significantly lower.
Table 1. Baseline characteristics of patients.
| Characteristics | PDS (n=175) | Early IDS (n=224) | DDS (n=150) | p-value | |
|---|---|---|---|---|---|
| Age (yr) | 58 (22–88) | 62 (21–82) | 63 (36–88) | 0.010 | |
| BMI (kg/m2) | 24.2 (16.5–44.1) | 24.0 (15.6–52.0) | 24.6 (15.6–43.9) | 0.484 | |
| Missing | 7 | 2 | 11 | ||
| WHO performance status classification | <0.001 | ||||
| 0 | 137 (79.2) | 122 (56.2) | 96 (65.3) | ||
| 1 | 32 (18.5) | 83 (38.2) | 44 (29.9) | ||
| ≥2 | 4 (2.3) | 12 (5.5) | 7 (4.8) | ||
| Missing | 2 | 7 | 3 | ||
| CA-125 (UI/mL) at diagnosis | 463 (7–23,762) | 800 (11–42,956) | 1,000 (5–86,000) | <0.001 | |
| Missing | 24 | 15 | 13 | ||
| FIGO stage | 0.002 | ||||
| IIIC | 158 (90.3) | 176 (78.6) | 115 (76.7) | ||
| IV | 17 (9.7) | 48 (21.4) | 35 (23.3) | ||
| Histological subtype | <0.001 | ||||
| Serous | 140 (80.0) | 207 (93.2) | 142 (95.3) | ||
| Non-serous | 35 (20.0) | 15 (6.8) | 7 (4.7) | ||
| Missing | 0 | 2 | |||
| Ascites (liter) at diagnosis) | 0.1 (0–7) | 1 (0–10) | 1 (0–8) | <0.001 | |
| Missing | 15 | 29 | 24 | ||
Values are presented as median (range) or number of patients (%). Bold-faced p-values indicate statistical significance.
BMI, body mass index; CA-125, cancer antigen-125; DDS, delayed debulking surgery; Early IDS, early interval debulking surgery; FIGO, International Federation of Gynaecology and Obstetrics; PDS, primary debulking surgery; WHO, World Health Organization.
All surgical data is displayed in Table 2. Median PCI at CRS was 11.5, 10 and 7 in the PDS, early IDS and DDS groups, respectively (p<0.001). Regarding surgical procedures, patients who received PDS had significantly more large bowel resections than patients who received debulking surgery either after 3–4 or 6 cycles of NACT. Women undergoing DDS had fewer diaphragm stripping and extended peritonectomies compared to patients after PDS or early IDS. The proportion of patients undergoing a CRS with an Aletti score ≥8 progressively decreased in the PDS, early IDS and DDS groups (p=0.006).
Table 2. Surgical data of patients.
| Variables | PDS (n=175) | Early IDS (n=224) | DDS (n=150) | p-value | |
|---|---|---|---|---|---|
| PCI | 11.5 (2–33) | 10 (0–39) | 7 (0–31) | <0.001 | |
| Missing | 3 | 1 | 2 | ||
| PCI | <0.001 | ||||
| PCI ≤10 | 76 (44.2) | 112 (50.2) | 99 (66.9) | ||
| PCI >10 | 96 (55.8) | 111 (49.8) | 49 (33.1) | ||
| Missing | 3 | 1 | 2 | ||
| Surgical procedures | |||||
| Hysterectomy | 167 (95.4) | 203 (90.6) | 121 (80.7) | <0.001 | |
| Unilateral or bilateral salpingoophorectomy | 164 (93.7) | 210 (93.8) | 128 (85.3) | 0.007 | |
| Pelvic lymphadenectomy | 158 (90.3) | 205 (91.5) | 132 (88.0) | 0.533 | |
| Aortic lymphadenectomy | 158 (90.3) | 201 (89.7) | 129 (86.0) | 0.412 | |
| Infragastric omentectomy | 173 (98.9) | 222 (99.1) | 145 (96.7) | 0.212 | |
| Small bowel resection | 20 (11.4) | 14 (6.3) | 10 (6.7) | 0.130 | |
| Large bowel resection | 96 (54.9) | 81 (36.2) | 48 (32.0) | <0.001 | |
| If large bowel resection, rectosigmoid resection (n=225) | 86 (89.6) | 73 (90.1) | 45 (93.8) | 0.798 | |
| Multiple bowel resection | 19 (10.9) | 19 (8.5) | 10 (6.7) | 0.405 | |
| Appendectomy | 93 (53.1) | 111 (49.6) | 74 (49.3) | 0.724 | |
| Right diaphragm stripping | 108 (61.7) | 150 (67.0) | 69 (46.0) | <0.001 | |
| Left diaphragm stripping | 56 (32.0) | 78 (34.8) | 29 (19.3) | 0.004 | |
| If diaphragm stripping, diaphragm resection (n=330) | 31 (28.7) | 26 (17.1) | 15 (21.4) | 0.083 | |
| Atypical hepatic resection | 4 (2.3) | 6 (2.7) | 5 (3.3) | 0.895 | |
| Cholecystectomy | 20 (11.4) | 19 (8.5) | 6 (4.0) | 0.051 | |
| Celiac lymph node resection | 24 (13.7) | 29 (12.9) | 12 (8.0) | 0.226 | |
| Splenectomy | 43 (24.6) | 53 (23.7) | 31 (20.7) | 0.687 | |
| Distal pancreatectomy | 6 (3.4) | 14 (6.3) | 11 (7.3) | 0.277 | |
| Partial gastrectomy | 5 (2.9) | 4 (1.8) | 2 (1.3) | 0.689 | |
| Extended peritonectomy | 89 (50.9) | 117 (52.2) | 50 (33.3) | <0.001 | |
| Glissonectomy | 15 (9.5) | 16 (8.3) | 15 (12.7) | 0.447 | |
| Mesentery or bowel vaporisation | 39 (22.3) | 59 (26.3) | 27 (18.0) | 0.166 | |
| Partial abdominal wall resection | 15 (8.6) | 53 (23.7) | 32 (21.3) | <0.001 | |
| Partial cystectomy or ureteral resection | 4 (2.3) | 2 (0.9) | 2 (1.3) | 0.509 | |
| Cardiophrenic lymph node resection | 1 (0.6) | 3 (1.3) | 6 (4.0) | 0.085 | |
| Inguinal lymph node resection | 4 (2.3) | 7 (3.1) | 2 (1.3) | 0.562 | |
| Axillary lymph node resection | 0 (0) | 1 (0.4) | 1 (0.7) | 0.739 | |
| CC-score | 0.567 | ||||
| CC-0 | 157 (89.7) | 195 (87.1) | 129 (86.0) | ||
| CC-1 | 18 (10.3) | 29 (12.9) | 21 (14.0) | ||
| Aletti score | 0.006 | ||||
| <8 | 80 (45.7) | 125 (55.8) | 95 (63.3) | ||
| ≥8 | 95 (54.3) | 99 (44.2) | 55 (36.7) | ||
| HIPEC | 0.001 | ||||
| No | 174 (99.4) | 223 (99.6) | 142 (94.7) | ||
| Yes | 1 (0.6) | 1 (0.4) | 8 (5.3) | ||
| IP chemotherapy | <0.001 | ||||
| No | 156 (89.1) | 224 (100) | 150 (100) | ||
| Yes | 19 (10.9) | 0 (0) | 0 (0) | ||
| Bevacizumab | <0.001 | ||||
| No | 143 (81.7) | 164 (73.2) | 135 (90.0) | ||
| Yes | 32 (18.3) | 60 (26.8) | 15 (10.0) | ||
Values are presented as median (range) or number of patients (%). Bold-faced p-values indicate statistical significance.
CC-score, Completeness of Cytoreduction score; DDS, delayed debulking surgery; Early IDS, early interval debulking surgery; Extended peritonectomy, peritonectomy of more than three abdominal regions; HIPEC, hyperthermic intraperitoneal chemotherapy; IP, intraperitoneal; PCI, peritoneal cancer index; PDS, primary debulking surgery; PH, porta hepatis; Partial abdominal wall resection, partial resection of anterior abdominal wall sheath, omphalectomy or port site resection.
The overall rate of major surgical complications (grade III–V) according to the Clavien-Dindo classification was higher after PDS (28.6%, 50/175) than after IDS at 3-4 cycles of NACT (23.2%, 52/224) or after DDS (14.0%, 21/150) (p=0.007). Table S1 shows the overall postoperative complications in the 3 groups. However, among women with a high Aletti score, the major surgical complication rate was not significantly different between the three groups: 35.8% (34/95) after PDS, 31.3% (31/99) after early IDS, and 18.2% (10/55) after DDS (p=0.073). Furthermore, among patients with a low Aletti score, the rate of major surgical complications was not significantly different in the three groups: 20.0% (16/80) for PDS, 16.8% (21/125) for early IDS and 11.6% (11/96) for DDS (p=0.302). Postoperative mortality rates were 1.1% (2/175) after PDS, 2.2% (5/224) after early IDS, and 2.0% (3/150) after DDS (p=0.723).
Median follow-up was 63.5 months (95% CI=59.6–70.5) in the PDS group, 62.6 months (95% CI=57.0–69.7) in the early IDS group, and 83.0 months (95% CI=68.1–90.1) in the DDS group. Median OS after PDS, IDS at 3–4 cycles and DDS groups were 84.0 months (95% CI=68.3–111.0), 50.7 months (95% CI=44.6–59.5) and 47.5 months (95% CI=39.3–52.9), respectively (p<0.001), without significant differences between the 2 groups of NACT (p=0.525). Among patients undergoing DDS, there was no survival difference between patients undergoing delayed IDS (median OS=47.5 months; 95% CI=39.3–52.4) and patients undergoing closure debulking surgery (median OS=51.8 months; 95% CI=25.9–104.9; p=0.612).
In multivariable analysis, the number of cycles of NACT (early IDS: HR=1.61; 95% CI=1.18–2.20; p=0.003 and DDS: HR=1.88; 95% CI=1.35–2.62; p<0.001, respectively), PCI >10 (HR=1.37; 95% CI=1.04–1.81; p=0.027), and Aletti score ≥8 (HR=1.36; 95% CI=1.03–1.79; p=0.028) were significantly associated with worse OS, while age (HR=1.01; 95% CI=1.00–1.02; p=0.082), WHO performance status ≥1 (HR=1.06; 95% CI=0.82–1.37; p=0.658), non-serous histological subtype (HR=1.33; 95% CI=0.90–1.96; p=0.157), International Federation of Gynaecology and Obstetrics (FIGO) stage IV (HR=0.95; 95% CI=0.69–1.31; p=0.772), CC-1 (HR=1.34; 95% CI=0.96–1.88; p=0.082), and maintenance treatment with bevacizumab (HR=0.93; 95% CI=0.68–1.29; p=0.673) were not significantly associated with OS.
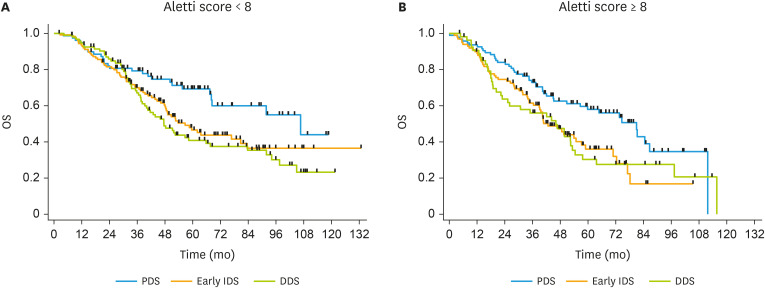
Table 3 shows OS according to surgical timing and surgical complexity measured with the Aletti score. In women with an Aletti score ≥8, median OS at PDS, early IDS and DDS was 80.5, 42.4, and 45.8 months (p=0.014), respectively; and in women with an Aletti score <8, median OS was 106.6, 56.7, and 47.5 months (p=0.013), respectively. No significant differences between the 2 groups of NACT were observed. In both subsets of patients with an Aletti score <8 and an Aletti score ≥8, the risk of death was increased after early IDS or DDS compared to upfront surgery. The hazard ratios for death with an Aletti score <8 increased progressively with the increasing number of cycles. Fig. 1 shows OS curves of PDS, early IDS and DDS in the subset of patients with an Aletti score <8 and ≥ 8.
Table 3. Analysis of OS according to surgical timing and Aletti surgical complexity score.
| Aletti score | PDS | Early IDS | DDS | |
|---|---|---|---|---|
| Aletti <8 | ||||
| Median OS (95%CI) (mo) | 106.6 (68.0–NR) | 56.7 (48.0–80.7) | 47.5 (39.3–65.8) | |
| HR (95% CI) | 1.00 (ref.) | 1.69 (1.08–2.66) | 1.96 (1.24–3.10) | |
| HRadj (95% CI) | 1.00 (ref.) | 1.79 (1.13–2.86) | 2.14 (1.32–3.46) | |
| Aletti ≥8 | ||||
| Median OS (95% CI) (mo) | 80.5 (50.5–86.1) | 42.4 (36.2–56.8) | 45.8 (22.8–52.9) | |
| HR (95% CI) | 1.00 (ref.) | 1.70 (1.15–2.53) | 1.69 (1.08–2.62) | |
| HRadj (95% CI) | 1.00 (ref.) | 1.66 (1.11–2.47) | 1.76 (1.12–2.78) | |
Bold-faced p-values indicate statistical significance.
CI, confidence interval; DDS, delayed debulking surgery; Early IDS, early interval debulking surgery; HR, hazard ratio; HRadj, adjusted hazard ratio for age, International Federation of Gynaecology and Obstetrics stage, peritoneal cancer index score and Completeness of Cytoreduction score; NR, not reached; OS, overall survival; PDS, primary debulking surgery.
Fig. 1. OS according to surgical timing in patients with an Aletti score <8 (A) and an Aletti score ≥8 (B).
DDS, delayed debulking surgery; Early IDS, early interval debulking surgery; OS, overall survival; PDS, primary debulking surgery.
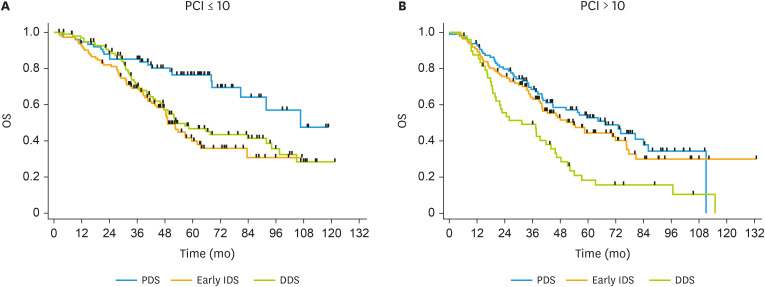
Table 4 shows OS according to surgical timing and tumor burden assessed by PCI. In women with PCI >10, median OS at PDS, early IDS and DDS was 67.4, 53.6, and 31.4 months (p<0.001), respectively. The difference was significant between early IDS and DDS (p=0.002) but not between PDS and early IDS (p=0.406). In women with PCI ≤10, median OS was 106.6, 49.2, and 52.9 months (p<0.001), respectively. No significant differences were observed between the two groups of NACT in this subset of patients. Among patients with PCI ≤10, the risk of death was increased after debulking surgery, either after 3–4 or 6 cycles of NACT when compared to PDS. Among patients with PCI >10, the risk of death was higher after DDS, but early IDS did not yield an increased risk of death. Fig. 2 displays OS curves of PDS, early IDS and DDS in the subset of patients with a PCI ≤10 and >10.
Table 4. Analysis of OS according to surgical timing and PCI.
| PCI | PDS | Early IDS | DDS | |
|---|---|---|---|---|
| PCI ≤10 | ||||
| Median OS (95% CI) (mo) | 106.6 (80.8–NR) | 49.2 (43.7–62.0) | 52.9 (46.0–91.9) | |
| HR (95% CI) | 1.00 (ref.) | 2.57 (1.56–4.23) | 2.09 (1.26–3.46) | |
| HRadj (95% CI) | 1.00 (ref.) | 2.55 (1.53–4.26) | 2.06 (1.23–3.46) | |
| PCI >10 | ||||
| Median OS (95% CI) (mo) | 67.4 (44.7–86.1) | 53.6 (40.0–76.6) | 31.4 (19.8–43.9) | |
| HR (95% CI) | 1.00 (ref.) | 1.17 (0.80–1.71) | 2.16 (1.42–3.29) | |
| HRadj (95% CI) | 1.00 (ref.) | 1.23 (0.84–1.80) | 2.07 (1.36–3.17) | |
Bold-faced p-values indicate statistical significance.
CI, confidence interval; DDS, delayed debulking surgery; Early IDS, early interval debulking surgery; HR, hazard ratio; HRadj, adjusted hazard ratio for age, International Federation of Gynaecology and Obstetrics stage, Aletti score and Completeness of Cytoreduction score; NR, not reached; OS, overall survival; PCI, peritoneal cancer index; PDS, primary debulking surgery.
Fig. 2. OS according to surgical timing in patients with PCI ≤10 (A) and PCI >10 (B).
DDS, delayed debulking surgery; Early IDS, early interval debulking surgery; OS, overall survival; PCI, peritoneal cancer index; PDS, primary debulking surgery.
DISCUSSION
We found a survival benefit of upfront surgery over NACT, with an additional 33 months of OS in patients undergoing PDS compared with patients undergoing early IDS or DDS. Our findings are concordant with several previous reports showing that upfront cytoreduction offers a survival benefit over IDS [4,5,29,30]. Hypothetically, delaying CRS after NACT promotes the selection of chemoresistant tumor cells, as the probability of developing chemoresistance increases with the increasing number of tumor cells. Therefore, even if IDS were to remove all macroscopic disease, the remaining microscopic residual tumor might have a reduced chemosensitivity [31]. In fact, Petrillo et al. demonstrated a worse disease behavior in terms of timing, pattern, and type of recurrence in patients undergoing IDS compared to patients treated with PDS, as patients treated with NACT more frequently presented platinum-resistant recurrences, carcinomatosis and a shorter platinum-free interval. These findings suggest that upfront surgery reduces the probability of development of resistant tumor clones [32]. However, due to the retrospective design of our series, patients in the PDS group might have had a better baseline prognosis as they were not selected for NACT, so this may have contributed to their improved survival.
Classically, when NACT is indicated, IDS is scheduled after three or four cycles of chemotherapy. However, some reports have assessed the impact of late IDS performed after more than four cycles of NACT [11,12,13,14]. In our study, there were no significant differences in survival between IDS at 3–4 cycles or at 6 cycles of NACT. Yoneoka et al. [11] found that in patients with non-resectable disease after three cycles of NACT, delivering 3 additional cycles before CRS offered a similar survival to that of patients undergoing IDS after 3 cycles of NACT. Similarly, Stoeckle et al. [14] found that survival of late IDS was not worse than with early IDS, and that the rate of complete cytoreduction was higher in patients undergoing late IDS. Other studies reported similar survival rates between patients receiving ≤4 or ≥5 cycles of NACT before IDS [12,13]. Our findings contrast with those of Colombo et al. [15], whose patients undergoing complete IDS after more than 4 cycles of NACT had a poorer prognosis. Xu et al. [16] reported an impaired oncological outcome of patients undergoing 5 or more cycles of NACT and they recommended not exceeding 4 cycles of NACT before IDS. A previous meta-analysis suggested that there is a gradual development of chemoresistant disease with the cumulative number of NACT cycles. However, most studies included patients undergoing suboptimal surgeries [5]. In contrast, our findings in the overall cohort suggest that when chemoresistant tumor cells have been selected by NACT, additional cycles do not impact survival. Another explanation could be that only patients with minimal or no residual disease were included in the present series.
In our study, surgical radicality was higher in women undergoing upfront surgery (54%) and decreased with the increasing number of cycles of NACT. The proportion of patients with an Aletti score ≥8 was 44% after IDS at 3–4 cycles and 37% after 6 cycles, although the difference was not significant. Surgical procedures such as diaphragmatic stripping, extended peritonectomy and large bowel resection were more frequent at PDS. This is concordant with previous studies as NACT is often associated with less extended surgical procedures and lower surgical morbi-mortality [8,10]. In a previous study, we showed that high surgical complexity according to the Aletti score was independently associated with a decreased disease-free survival [6]. In the present study, median OS decreased with the increasing number of NACT cycles in patients with an Aletti score <8. The difference in median OS between PDS and early or delayed IDS among patients with an Aletti score <8 was 50 and almost 60 months, respectively. In patients undergoing more radical surgeries (Aletti score ≥8), the benefit of PDS over early or delayed IDS was lower (38 and 35 months, respectively). In other words, the negative impact of a high Aletti score decreased with NACT. The difference in median OS between Aletti ≥8 and Aletti <8 after PDS was 26 months, while this difference was about 14 and less than 2 months after early and delayed IDS, respectively.
Little is known regarding the effect of radical surgery on the survival benefit offered by upfront surgery. Our results contrast with a previous study which did not show a significantly different prognostic outcome between patients receiving radical or simple upfront surgical procedures [21]. This contradiction may be explained by different definitions of radical surgery, as in the current study we assessed surgical complexity with Aletti score, which includes different items to define radical surgery [27]. Extended peritoneal disease requiring ultraradical procedures probably has an adverse tumor biology and surgical efforts may not completely overcome this deleterious effect [33]. Even if the benefit of PDS is impaired by high surgical radicality, we still found a survival advantage of upfront surgery with complete cytoreduction over IDS of almost 40 months in these patients. PDS should remain the mainstay of surgical treatment, even when complex procedures are required to achieve microscopic or no residual tumor.
Concordantly with previous reports, we found that NACT was associated with lower morbidity, 29% after PDS versus 23% after early IDS and 14% after DDS [7,8]. However, among patients with high surgical complexity, the rate of major surgical complications was not associated with NACT.
No residual tumor is widely recognized as the most powerful predictor of clinical outcome in advanced OC [2,34]. However, even in the event of complete cytoreduction, intraabdominal tumor burden still has a negative impact on survival [6,33]. Tentes et al. [35] demonstrated that PCI accurately reflects peritoneal spread and disease burden in advanced OC patients. Some studies have reported that PCI is an independent prognostic factor and that a cut-off value above 10 negatively impacts survival [6,35,36]. Moreover, in the subset of patients with no residual disease, high PCI scores remain associated with poor survival rates [6,37]. Even though the benefit of optimal cytoreduction has been shown to decrease with increasing tumor volume, there is still a significant survival benefit conferred by complete CRS in patients with high disease burden [33]. The impact of high tumor burden on survival according to the number of NACT cycles is unknown. To our knowledge, this is the first study to assess this issue.
In our study, median PCI score at CRS progressively decreased in PDS, early IDS and delayed IDS. The OS advantage of upfront CRS compared to NACT was enhanced in patients with low tumor burden. In this group, there was a survival difference of more than 50 months between upfront surgery and the NACT groups. In addition, our results concordantly showed that among patients with high disease burden, there was no survival difference between PDS and early IDS. Our findings are concordant with the recent randomised SCORPION trial, which included only patients with high tumor load. It reported that PDS and NACT with 3–4 cycles had superimposable survival outcomes in this subset of patients [18]. However, in our study, DDS after 6 cycles of NACT in patients with high PCI at CRS yielded the worst survival rates. Owing to the retrospective nature of our study, it is unclear whether the inferior clinical outcome of this subgroup was due to chemoresistance induced by additional cycles of NACT or to the selection of patients with a poorer prognosis who were not considered good surgical candidates after 3–4 cycles of NACT. In addition, among the patients who underwent DDS, there was no survival difference between those who had delayed IDS and those undergoing closure debulking surgery. It is unclear whether adjuvant chemotherapy after DDS did not improve clinical outcome or if patients receiving adjuvant chemotherapy had a worse prognosis due to the poor response to NACT.
The strengths of our study include a large homogeneous cohort with more than 500 patients with minimal or no residual disease after CRS and a long-term follow-up. Surgical complexity and tumor load were assessed using validated and objective systems such as the Aletti score and PCI [25,27]. The main weakness of the study is its retrospective design with the intrinsic risk of selection bias. As NACT was indicated in medically non-operable patients or in case of non-resectable carcinomatosis, patients with more extended disease were probably included in early IDS and DDS groups, which might have influenced our results. Another important limitation is that PCI was collected at CRS instead of at diagnosis, which would have allowed a more reliable comparison of tumor load between the 3 groups. Unfortunately, although our patients systematically underwent laparoscopy at diagnosis, PCI at this surgery was not available in the surgical report for most patients. The unexpected and uncontrolled significant differences in the baseline characteristics between the three groups could have influenced these findings. The role of early and delayed IDS according to surgical complexity and disease burden needs to be confirmed in prospective multicentric studies.
In conclusion, PDS is associated with higher survival rates than early or delayed IDS. The survival benefit of PDS over NACT is higher in women requiring less complex surgeries, even if radical upfront procedures are still associated with a survival advantage over NACT. Similarly, low tumor load at CRS enhances the survival benefit of PDS over early or delayed IDS. In patients with high tumor load, delayed IDS yields impairs the oncological outcome.
ACKNOWLEDGEMENTS
The project that gave rise to these results received the support of a fellowship from “la Caixa” Foundation (ID 100010434). The fellowship code is LCF/BQ/EU18/11650038.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: A.M.A., C.B., G.A., P.A., S.E., R.A., M.C., G.F., Z.I., Q.D., I.C., M.F., B.S., F.G., H.A., M.A.
- Data curation: A.M.A., C.B., S.E., R.A., M.C.
- Formal analysis: C.B.
- Investigation: A.M.A., C.B., G.A., P.A., S.E., R.A., M.C., G.F., Z.I., Q.D., I.C., M.F., B.S., F.G., H.A., M.A.
- Methodology: A.M.A., C.B., G.A., P.A., S.E., R.A., M.C., G.F., Z.I., Q.D., I.C., M.F., B.S., F.G., H.A., M.A.
- Project administration: A.M.A., C.B., G.A., P.A., S.E., R.A., M.C., G.F., Z.I., Q.D., I.C., M.F., B.S., F.G., H.A., M.A.
- Resources: A.M.A., C.B., G.A., P.A., S.E., R.A., M.C., G.F., Z.I., Q.D., I.C., M.F., B.S., F.G., H.A., M.A.
- Software: C.B.
- Supervision: H.A., M.A.
- Validation: A.M.A., C.B., G.A., P.A., S.E., R.A., M.C., G.F., Z.I., Q.D., I.C., M.F., B.S., F.G., H.A., M.A.
- Visualization: A.M.A., C.B., G.A., P.A., S.E., R.A., M.C., G.F., Z.I., Q.D., I.C., M.F., B.S., F.G., H.A., M.A.
- Writing - original draft: A.M.A.
- Writing - review & editing: C.B., G.A., P.A., S.E., R.A., M.C., G.F., Z.I., Q.D., I.C., M.F., B.S., F.G., H.A., M.A.
SUPPLEMENTARY MATERIALS
Postoperative complications according to Clavien-Dindo classification
Flow chart of the eligible and the included patients.
References
- 1.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 2.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 3.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 4.Chiva L, Lapuente F, Castellanos T, Alonso S, Gonzalez-Martin A. What should we expect after a complete cytoreduction at the time of interval or primary debulking surgery in advanced ovarian cancer? Ann Surg Oncol. 2016;23:1666–1673. doi: 10.1245/s10434-015-5051-9. [DOI] [PubMed] [Google Scholar]
- 5.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103:1070–1076. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Angeles MA, Rychlik A, Cabarrou B, Spagnolo E, Guyon F, Pérez-Benavente A, et al. A multivariate analysis of the prognostic impact of tumor burden, surgical timing and complexity after complete cytoreduction for advanced ovarian cancer. Gynecol Oncol. 2020;158:614–621. doi: 10.1016/j.ygyno.2020.06.495. [DOI] [PubMed] [Google Scholar]
- 7.Wright AA, Bohlke K, Armstrong DK, Bookman MA, Cliby WA, Coleman RL, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34:3460–3473. doi: 10.1200/JCO.2016.68.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartels HC, Rogers AC, McSharry V, McVey R, Walsh T, O'Brien D, et al. A meta-analysis of morbidity and mortality in primary cytoreductive surgery compared to neoadjuvant chemotherapy in advanced ovarian malignancy. Gynecol Oncol. 2019;154:622–630. doi: 10.1016/j.ygyno.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Vergote I, Coens C, Nankivell M, Kristensen GB, Parmar MKB, Ehlen T, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018;19:1680–1687. doi: 10.1016/S1470-2045(18)30566-7. [DOI] [PubMed] [Google Scholar]
- 10.Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer. 2016;64:22–31. doi: 10.1016/j.ejca.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Yoneoka Y, Ishikawa M, Uehara T, Shimizu H, Uno M, Murakami T, et al. Treatment strategies for patients with advanced ovarian cancer undergoing neoadjuvant chemotherapy: interval debulking surgery or additional chemotherapy? J Gynecol Oncol. 2019;30:e81. doi: 10.3802/jgo.2019.30.e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akladios C, Baldauf JJ, Marchal F, Hummel M, Rebstock LE, Kurtz JE, et al. Does the number of neoadjuvant chemotherapy cycles before interval debulking surgery influence survival in advanced ovarian cancer? Oncology. 2016;91:331–340. doi: 10.1159/000449203. [DOI] [PubMed] [Google Scholar]
- 13.Phillips A, Sundar S, Singh K, Nevin J, Elattar A, Kehoe S, et al. Complete cytoreduction after five or more cycles of neo-adjuvant chemotherapy confers a survival benefit in advanced ovarian cancer. Eur J Surg Oncol. 2018;44:760–765. doi: 10.1016/j.ejso.2018.01.097. [DOI] [PubMed] [Google Scholar]
- 14.Stoeckle E, Boubli B, Floquet A, Brouste V, Sire M, Croce S, et al. Optimal timing of interval debulking surgery in advanced ovarian cancer: yet to be defined? Eur J Obstet Gynecol Reprod Biol. 2011;159:407–412. doi: 10.1016/j.ejogrb.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Colombo PE, Labaki M, Fabbro M, Bertrand M, Mourregot A, Gutowski M, et al. Impact of neoadjuvant chemotherapy cycles prior to interval surgery in patients with advanced epithelial ovarian cancer. Gynecol Oncol. 2014;135:223–230. doi: 10.1016/j.ygyno.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Deng F, Lv M, Chen X. The number of cycles of neoadjuvant chemotherapy is associated with prognosis of stage IIIc-IV high-grade serous ovarian cancer. Arch Gynecol Obstet. 2017;295:451–458. doi: 10.1007/s00404-016-4256-x. [DOI] [PubMed] [Google Scholar]
- 17.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Fagotti A, Ferrandina MG, Vizzielli G, Pasciuto T, Fanfani F, Gallotta V, et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION- NCT01461850) Int J Gynecol Cancer. 2020;30:1657–1664. doi: 10.1136/ijgc-2020-001640. [DOI] [PubMed] [Google Scholar]
- 19.Chi DS, Zivanovic O, Levinson KL, Kolev V, Huh J, Dottino J, et al. The incidence of major complications after the performance of extensive upper abdominal surgical procedures during primary cytoreduction of advanced ovarian, tubal, and peritoneal carcinomas. Gynecol Oncol. 2010;119:38–42. doi: 10.1016/j.ygyno.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Gerestein CG, Damhuis RA, Burger CW, Kooi GS. Postoperative mortality after primary cytoreductive surgery for advanced stage epithelial ovarian cancer: a systematic review. Gynecol Oncol. 2009;114:523–527. doi: 10.1016/j.ygyno.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Martinez A, Ngo C, Leblanc E, Gouy S, Luyckx M, Darai E, et al. Surgical complexity impact on survival after complete cytoreductive surgery for advanced ovarian cancer. Ann Surg Oncol. 2016;23:2515–2521. doi: 10.1245/s10434-015-5069-z. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz NS, Miller A, Rungruang B, Richard SD, Rodriguez N, Bookman MA, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. 2015;33:937–943. doi: 10.1200/JCO.2014.56.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford SC, Vasey PA, Paul J, Hay A, Davis JA, Kaye SB. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. J Clin Oncol. 2005;23:8802–8811. doi: 10.1200/JCO.2005.02.1287. [DOI] [PubMed] [Google Scholar]
- 24.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilly FN, Cotte E, Brigand C, Monneuse O, Beaujard AC, Freyer G, et al. Quantitative prognostic indices in peritoneal carcinomatosis. Eur J Surg Oncol. 2006;32:597–601. doi: 10.1016/j.ejso.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med. 2019;380:822–832. doi: 10.1056/NEJMoa1808424. [DOI] [PubMed] [Google Scholar]
- 27.Aletti GD, Santillan A, Eisenhauer EL, Hu J, Aletti G, Podratz KC, et al. A new frontier for quality of care in gynecologic oncology surgery: multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol Oncol. 2007;107:99–106. doi: 10.1016/j.ygyno.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Y, Xie S, Zhang N, Wang J, Lv C, Guo J, et al. Platinum-based neoadjuvant chemotherapy versus primary surgery in ovarian carcinoma International Federation of Gynecology and Obstetrics stages IIIc and IV: a systematic review and meta-analysis. Gynecol Obstet Invest. 2018;83:209–219. doi: 10.1159/000485618. [DOI] [PubMed] [Google Scholar]
- 30.Qin M, Jin Y, Ma L, Zhang YY, Pan LY. The role of neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer: a systematic review and meta-analysis of randomized controlled trials and observational studies. Oncotarget. 2017;9:8614–8628. doi: 10.18632/oncotarget.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooke SL, Brenton JD. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol. 2011;12:1169–1174. doi: 10.1016/S1470-2045(11)70123-1. [DOI] [PubMed] [Google Scholar]
- 32.Petrillo M, Ferrandina G, Fagotti A, Vizzielli G, Margariti PA, Pedone AL, et al. Timing and pattern of recurrence in ovarian cancer patients with high tumor dissemination treated with primary debulking surgery versus neoadjuvant chemotherapy. Ann Surg Oncol. 2013;20:3955–3960. doi: 10.1245/s10434-013-3091-6. [DOI] [PubMed] [Google Scholar]
- 33.Zivanovic O, Sima CS, Iasonos A, Hoskins WJ, Pingle PR, Leitao MM, Jr, et al. The effect of primary cytoreduction on outcomes of patients with FIGO stage IIIC ovarian cancer stratified by the initial tumor burden in the upper abdomen cephalad to the greater omentum. Gynecol Oncol. 2010;116:351–357. doi: 10.1016/j.ygyno.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 35.Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol. 2013;130:493–498. doi: 10.1016/j.ygyno.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 36.Tentes AA, Tripsiannis G, Markakidis SK, Karanikiotis CN, Tzegas G, Georgiadis G, et al. Peritoneal cancer index: a prognostic indicator of survival in advanced ovarian cancer. Eur J Surg Oncol. 2003;29:69–73. doi: 10.1053/ejso.2002.1380. [DOI] [PubMed] [Google Scholar]
- 37.Llueca A, Escrig J MUAPOS working group (Multidisciplinary Unit of Abdominal Pelvic Oncology Surgery) Prognostic value of peritoneal cancer index in primary advanced ovarian cancer. Eur J Surg Oncol. 2018;44:163–169. doi: 10.1016/j.ejso.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Gasimli K, Braicu EI, Richter R, Chekerov R, Sehouli J. Prognostic and predictive value of the peritoneal cancer index in primary advanced epithelial ovarian cancer patients after complete cytoreductive surgery: study of Tumor Bank Ovarian Cancer. Ann Surg Oncol. 2015;22:2729–2737. doi: 10.1245/s10434-014-4329-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Postoperative complications according to Clavien-Dindo classification
Flow chart of the eligible and the included patients.