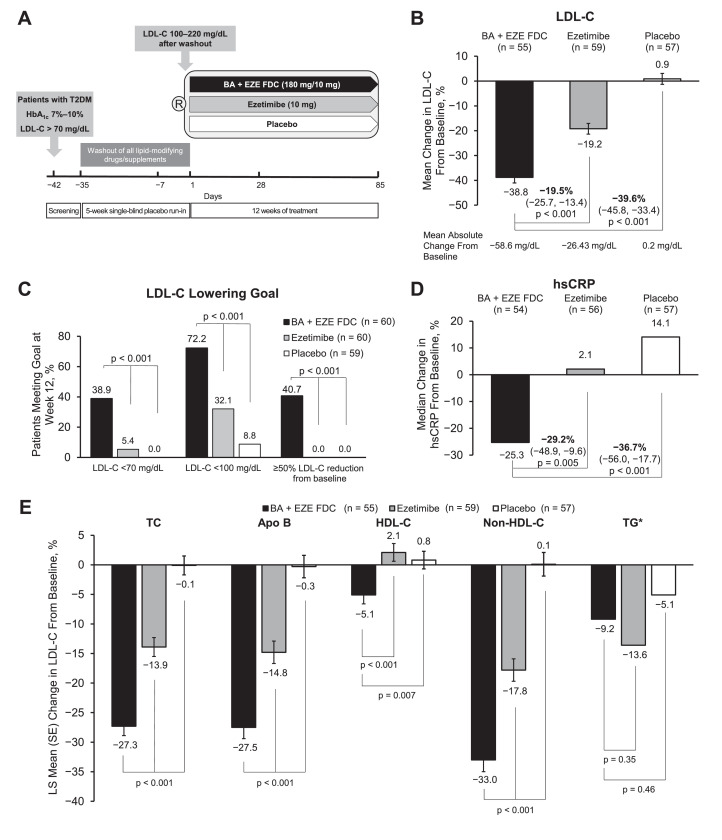
Fig. 1.
Study design and efficacy of BA + EZE FDC in patients with T2DM. (A) Schematic of the study design. (B) Percent change from baseline in LDL-C levels at week 12. (C) Proportion of patients meeting LDL-C lowering goals at week 12. (D) Percent change from baseline in hsCRP levels at week 12. (E) Percent change from baseline in TC, Apo B, HDL-C, non-HDL-C, and TG levels at week 12. *Data for TG are presented as median; interquartile range is 47.4 mg/dL for BA + EZE FDC (n = 54), 29.9 mg/dL for ezetimibe (n = 56), and 32.0 mg/dL for placebo (n = 57). Bars represent the standard error (SE) of the least squares mean. Abbreviations: Apo B, apolipoprotein B; BA + EZE FDC, bempedoic acid plus ezetimibe fixed-dose combination; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; R, randomization; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides.