Figure 4.
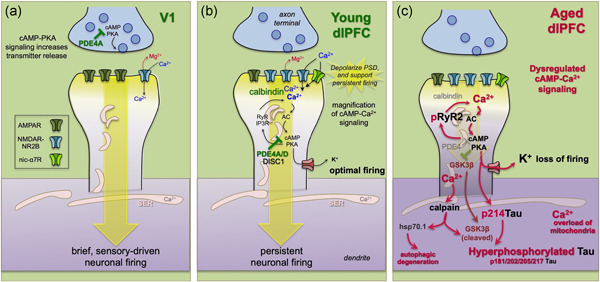
Neurotransmission and neuromodulation in rhesus monkey V1 versus dlPFC, and its dysregulation with advancing age. (a) V1 neurons show classic characteristics, with neurotransmission relying heavily on AMPAR. In layer III of V1, PDE4A regulation of cAMP signaling is concentrated in presynaptic glutamatergic terminals, where cAMP‐PKA signaling can enhance glutamate release. Consistent with the immunoEM, physiological studies show that increasing cAMP‐PKA signaling in V1 increases neuronal firing. (b) Neurotransmission and neuromodulation are very different in layer III of the dlPFC, where neurotransmission relies heavily on NMDAR, including those with slowly closing NR2B subunits that flux high levels of calcium. PDE4 is concentrated in layer III spines on the SER, positioned to regulate cAMP‐PKA drive on internal calcium release, which in turn increases cAMP production, creating a vicious cycle that must be tightly regulated by PDE4s. While moderate levels of calcium are necessary to sustain persistent firing, higher levels of cAMP‐calcium signaling open nearby potassium (K+) channels to weaken connectivity and reduce firing, for example, as occurs with uncontrollable stress. In young adult dlPFC, layer III dlPFC pyramidal cells express high levels of PDE4s to regulate cAMP drive on calcium release, and calbindin to regulate calcium levels when it is released into the cytosol. These regulatory factors are lost with advanced age. (c) The loss of PDE4s and calbindin in aged dlPFC pyramidal cells leads to excessive calcium‐cAMP‐PKA signaling and initiates a series of vicious cycles and toxic events including: (1) PKA phosphorylation of RyR2 to cause calcium leak into the cytosol, leading to more cAMP‐PKA signaling; (2) excessive opening of K+ channels that reduce neuronal firing; (3) calcium overload of mitochondria to induce inflammatory signaling (see Figure 6); (4) PKA phosphorylation of tau, which primes tau for hyperphosphorylation by GSK3β; (5) with sufficient cytosolic calcium, the activation of calpain, which disinhibits GSK3β to hyperphosphorylate tau at key sites that lead to tau fibrillation; and (6) calpain activation of heatshock protein 70.1 to drive autophagic degeneration. dlPFC, dorsolateral prefrontal association cortex
