Figure 1:
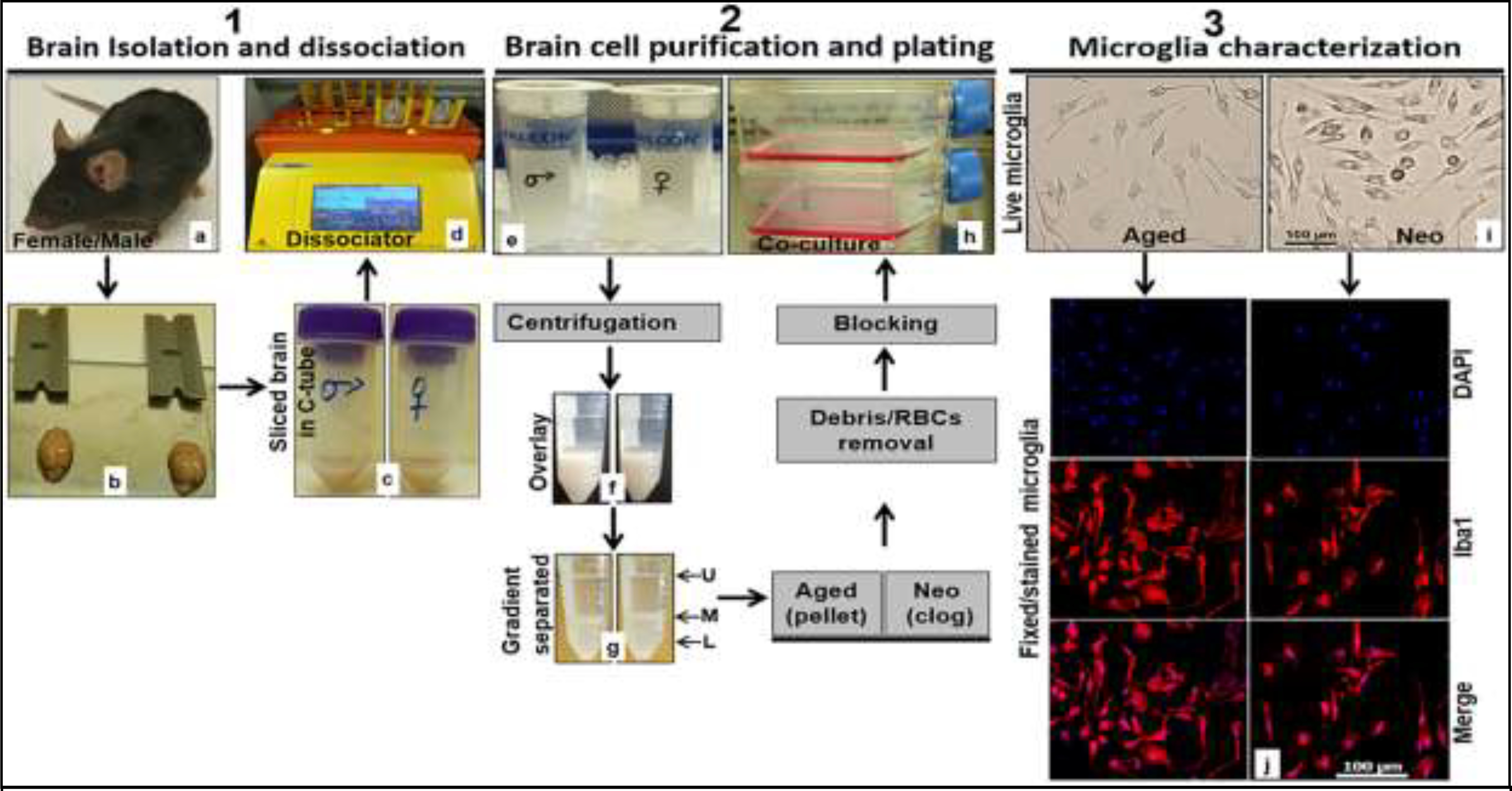
Workflow chart to isolate, culture and characterize microglia from aged and neonatal C57BL/6 mice. 1.1. (a) C57BL/6 mouse, (b) brain isolated from mouse skull ready for physical slicing, (c) sliced brain in C-tube containing neural tissue dissociation buffer, (d) gentleMACS Octo Dissociator with Heaters loaded with 2 C-tubes containing sliced brains and in neural tissue dissociation buffer. 1.2. (e) Straining of dissociated cells into single cells using a cell strainer, (f) gradient density overlaying of dissociated brain tissue, (g) phase separated cell suspension (L lower phase, M middle phase, and U upper phase) after centrifugation, (h) seeding of brain cells in growth medium after debris and red blood cell removal steps. 1.3. (i) Representative live microglial cells taken in EVOS FL Auto 2 light microscope at 20x, (j) representative formaldehyde fixed and dye-stained microglia taken in Leica DMi8 Confocal Microscope at 20x (DAPI blue, Iba1 Red). RBCs, red blood cells.
