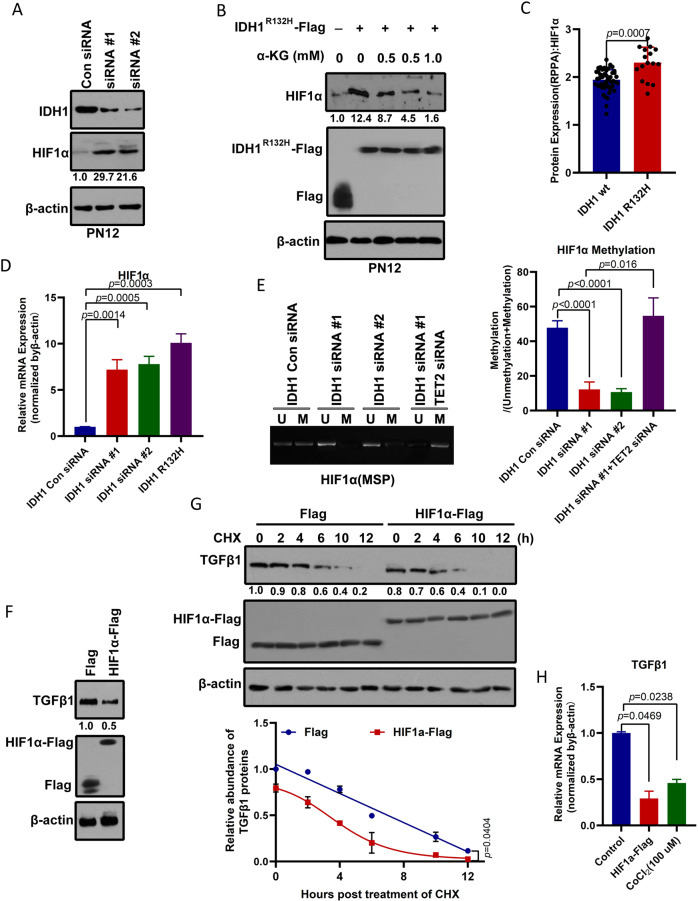
Fig. 1. IDH1 mutation mediates induction of HIF-1α and TGF-β1 protein stability in proneural GSCs.
A IDH1 knockdown elevates HIF-1α levels in the proneural GSC subtype, PN12. IDH1 and HIF-1α protein levels were determined by western blotting from PN12 GSCs transduced with control or siRNA targeting IDH1. The relative intensity (HIF-1α/β-actin) was quantified and indicated below. B A cell-permeable α-KG derivative blocks induction of HIF-1α in cells expressing IDH1 R132H. PN12 GSCs were transfected with IDH1 R132H, and different concentrations of octyl-α-KG ester were added to each transfected cell for 4 h. HIF-1α protein levels were assayed by western blotting. The relative intensity (HIF-1α/β-actin) was quantified and is indicated below. C Analysis of reverse-phase protein array expression data for proneural GBM from The Cancer Genome Atlas containing data for 64 tumor samples. HIF-1α protein levels were upregulated in IDH1 R132H mutant GBM (n = 16) when compared with wild-type IDH1 GBM (n = 48). Differences between groups were examined for statistical significance by the Student’s t-test using GraphPad Prism software, and the log-rank p-value was used to determine statistical significance, without multiple testing correction. D IDH1 knockdown or mutant IDH1 increases HIF-1α mRNA. After PN12 GSCs were transfected with control or siRNA targeting IDH1 or with the IDH1 R132H mutant, expression of HIF-1α mRNA was measured by quantitative polymerase chain reaction. E IDH1 knockdown reduces methylation of HIF-1α. Methylation-specific PCR (MSP) measurement of DNA methylation levels of the HIF-1α gene in PN12 GSCs. M and U represent amplification of methylated and unmethylated portions, respectively. Quantification of MSP band density for methylated alleles (closed bars) and unmethylated alleles (open bars) is shown. F Overexpression of HIF-1α reduced TGF-β1 levels in proneural GSCs, PN12. HIF-1α and TGF-β1 protein levels were determined by western blotting from PN12 GSCs transduced with control or Flag-tagged HIF-1α. The relative intensity (TGF-β1/β-actin) was quantified and is indicated below. G HIF-1α reduces the stability of TGF-β1 protein. PN12 GSCs were transfected with Flag-tagged HIF-1α for 24 h and then incubated with cyclohexidine 200 μg/ml, after which the cells were harvested at the indicated times for Western blot analysis. Relative TGF-β1 protein levels normalized to β-actin are presented relative to the level (set as 1.0) at 0 h post-CHX treatment (upper panel). H HIF-1α decreases expression of TGF-β1 mRNA. After PN12 was transfected with Flag-tagged HIF-1α or treated with CoCl2 (100 μmol/l) for 24 h, expression of TGF-β1 mRNA was measured by quantitative polymerase chain reaction. Analysis of variance coupled with Dunnett’s test (two-sided) was used for post hoc comparisons. *p < 0.05 versus the control group. GBM glioblastoma, GSCs glioblastoma stem cells, HIF-1α hypoxia-inducible factor 1-alpha, IDH1 isocitrate dehydrogenase 1.