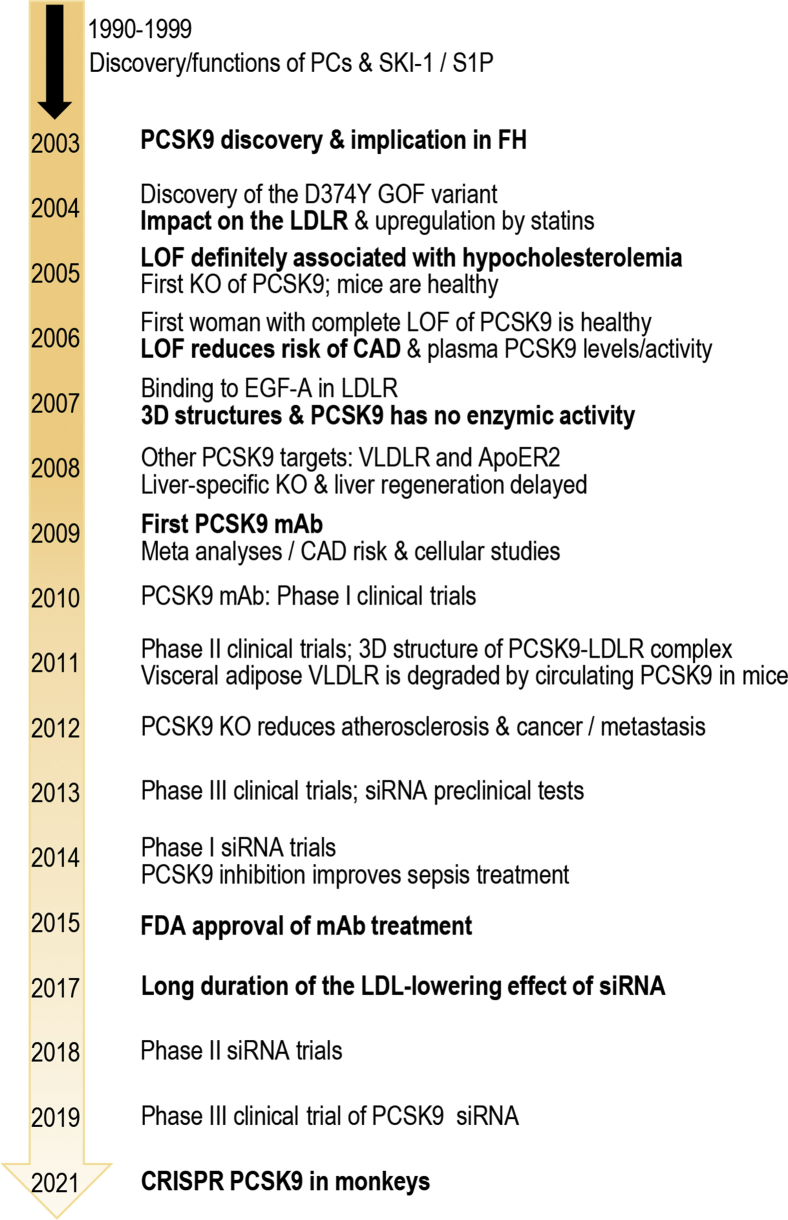
Fig. 5.
Time line of the critical discovery of PCSK9, its biological functions, the clinical use of either mAb or siRNA treatments, and the preclinical evaluation of a CRISPR approach to delete the gene. The first seven basic amino acid-specific PCs and SKI-1/S1P were identified and characterized during the period of 1990–1999. The complete sequence of human, rat, and mouse PCSK9 was first reported in 2003, as well as the association of the two GOF variants S127R and F216L with FH. The various time lines for the definition of PCSK9 activity and mechanism of function in cells, mouse models, and human ultimately led to the development of potent and safe inhibitory mAb (evolocumab/Repatha and alirocumab/Praluent) following various clinical trials, culminating in 2015–2016 with the Food and Drug Administration approval of the first mAb for clinical use in hypercholesterolemia treatments to reduce coronary artery disease (CAD). The use of liver-targeted nanoparticle carrying a PCSK9 siRNA (inclisiran) received marketing authorization in the European Union in December 2020 and should soon be ready for approval by the Food and Drug Administration. Preclinical evaluations of CRISPR editing of PCSK9 in monkeys were reported (114).