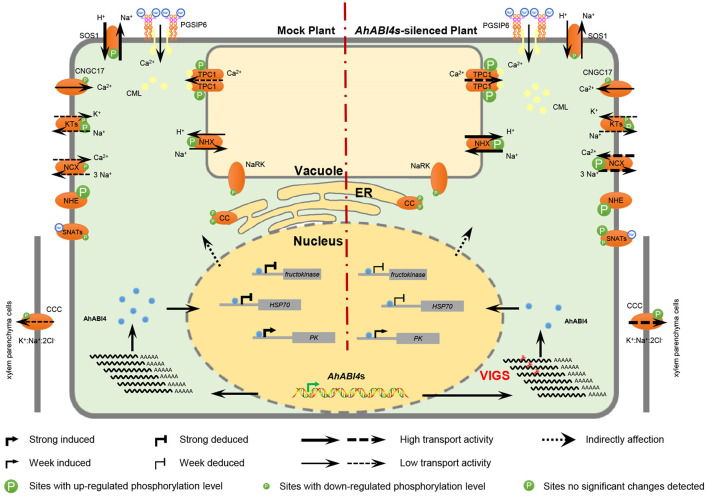
Figure 6.
Hypothetical model of ion homeostasis regulated by AhABI4s under salt-stress. Virus-induced gene silencing downregulated AhABI4s and influenced the expression of downstream targets (nucleus). Changes in protein levels of the downstream targets altered protein-protein interactions, which further lead to phosphorylation level and phosphorylation sites alteration in ion transporter/channel. Predicted peanut Na+ sensor PGSIP6 detected extracellular Na+ and gated Ca2+ influx channel on the plasma membrane to increase cytoplasmic Ca2+ content. On the other hand, PGSIP6 may also induce the transporting of vacuole Ca2+ into the cytoplasm via interaction with CMLs. Silencing of AhABI4s facilitated this process by promoting CML accumulation and TPC1 phosphorylation. Elevated Ca2+ content triggered a cellular defensive response to salt-stress. Na+ in the cytoplasm was transported into vacuoles and out of cells by NHX and NCX, respectively. In AhABI4s-silenced plants, NHX and NCX phosphorylation levels were higher than those in Mock plants and corresponded to relatively higher ion transport activity. Excess Na+ transported out of cytoplasm was transported into xylem parenchyma cells by CCC protein. Comparatively reduced cytoplasmic Na+ content in AhABI4s-silenced plants explained their salt tolerance phenotype. Solid and broken arrows represent proven and predicted functions, respectively.