Table 15.
Enantioselective 1,6-aza-Michael addition of isoxazolin-5-ones to p-quinone methides.
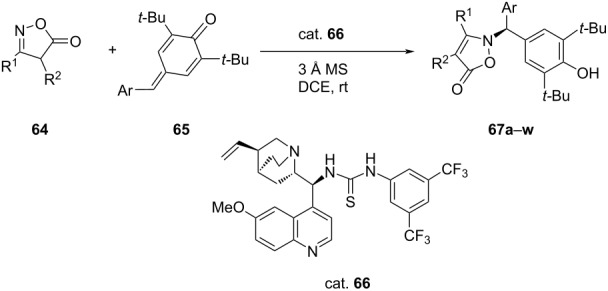
| |||||
|
| |||||
| 67 | R1 | R2 | Ar | Yield [%] | ee [%]a |
|
| |||||
| a | Me | H | Ph | 65 | 87 |
| b | Et | H | Ph | 51 | 81 |
| c | Pr | H | Ph | 50 | 81 |
| d | Ph | H | Ph | 77 | 54 |
| e | Pr | H | Ph | 78 | 89 |
| f | Me | Me | Ph | 66 | 62 |
| g | Me | H | p-MeC6H4 | 62 | 88 |
| h | Me | H | p-MeOC6H4 | 74 | 84 |
| i | Me | H | p-ClC6H4 | 43 | 48 |
| j | Me | H | p-O2NC6H4 | 47 | 89 |
| k | Me | H | o-MeOC6H4 | 81 | 94 |
| l | Me | H | o-ClC6H4 | 94 | 96 |
| m | Me | H | o-BrC6H4 | 43 | 90 |
| n | Me | H | m-MeOC6H4 | 20 | 25 |
| o | Me | H | m-ClC6H4 | 36 | 81 |
| p | Me | H | m-O2NC6H4 | 56 | 77 |
| q | Pr | H | p-MeOC6H4 | 75 | 79 |
| r | Pr | H | p-ClC6H4 | 78 | 88 |
| s | Pr | H | p-O2NC6H4 | 80 | 86 |
| t | Pr | H | o-ClC6H4 | 76 | 92 |
| u | Pr | H | m-MeOC6H4 | 82 | 82 |
| v | Pr | H | m-ClC6H4 | 80 | 88 |
| w | Pr | H | Ph | 71 | 86 |
aDetermined by HPLC using a chiral stationary phase.
