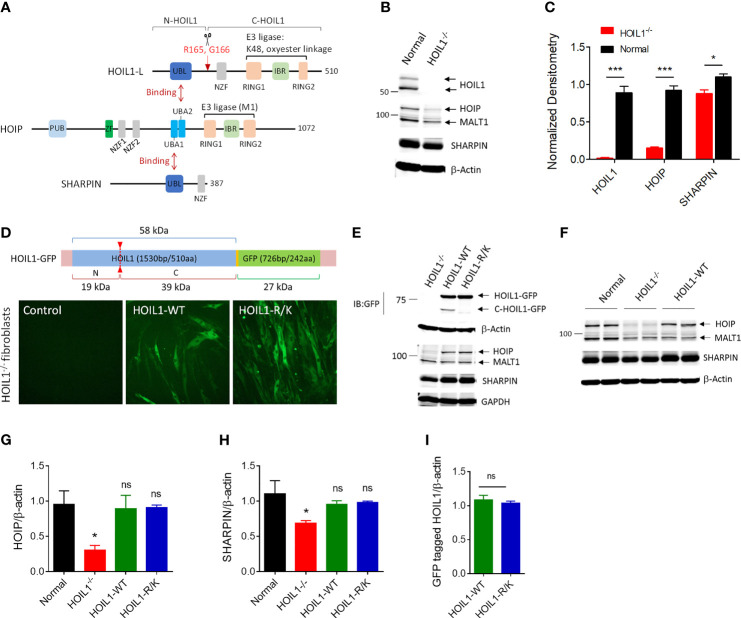
Figure 1.
Construction and characterization of human cells expressing a MALT1-resistant non-cleavable HOIL1 (HOIL1-R/K). (A) A schematic diagram illustrating the structural domains of HOIL1-L, HOIP and SHARPIN forming the LUBAC. HOIL1-L, active isoform of HOIL1 in the LUBAC, is cleaved at R165 by MALT1 to produce N- and C-terminal fragments, N-HOIL1 and C-HOIL1, respectively. The UBL domain of HOIL1 binds to the UBA2 domain of HOIP, whereas the UBL domain of SHARPIN binds to the UBA1 domain of HOIP, forming a stable trimolecular complex. (B) Representative immunoblots showing the expression levels of HOIL1, HOIP, SHARPIN and MALT1 in control and HOIL1-deficient (HOIL1–/–) human fibroblasts. (C) Normalized (to β-actin) densitometry analyses of HOIL1, HOIP and SHARPIN protein levels from (A) (N ≥ 3). (D) Top: schematic representation of the HOIL1-GFP constructs used. Bottom: micrographs demonstrating transduction of GFP-tagged wild-type (WT) or non-cleavable (R/K) HOIL1 in HOIL1–/– human fibroblasts. (E) Immunoblots showing transduction of different HOIL1-GFP constructs affecting HOIL1 cleavage capability and expression of HOIP, SHARPIN, and MALT1. (F) Immunoblots demonstrating the influence of HOIL1 expression on the stability of the other LUBAC components HOIP and SHARPIN compared to normal human fibroblasts. β-actin as the internal control. (G, H) Densitometry quantification of HOIP (G) and SHARPIN (H) levels in the four conditions: normal fibroblasts, HOIL1–/– fibroblasts, HOIL1–/– fibroblasts expressing HOIL1-WT and HOIL1–/– fibroblasts expressing HOIL1-R/K (N ≥ 3). (I) Densitometry quantification of GFP-tagged HOIL1-WT and HOIL1-R/K expressed in HOIL1–/– fibroblasts after transduction (N ≥ 3). *p < 0.05, ***p < 0.001, ns, non-significant relative to control unless otherwise indicated.