Abstract

The following salts have been synthesized and structurally characterized: Na2[IrF6]·2H2O (C2/m, a = 6.6327(4), b = 10.0740(6), c = 5.9283(5) Å, β = 122.3880(10)°) and Na3[IrF6]·2H2O (R-3, a = 7.5963(3), b = 7.5963(3), c = 9.8056(4) Å) (for the first time) by single-crystal X-ray diffraction; the unit cell parameters of a tetragonal phase (P42/mnm, a = 5.005(2), c = 10.074(4) Å) of the stable α-Na2[IrF6] were determined for the first time; and the unit cell parameters of β-Na2[IrF6] (P321, a = 9.332(4), c = 5.136(2) Å) and Na3[IrF6] (P21/n, a = 5.567(4), b = 5.778(4), c = 8.017(2) Å, β = 90.41(2)°) were determined using powder X-ray diffraction (PXRD). The data of the thermal stability was obtained by differential thermal analysis (DTA) for all substances. The presence of Na3[IrF6]·H2O monohydrate is predicted. H2[IrF6] was prepared in a solution and was demonstrated to behave as a strong dibasic acid.
1. Introduction
Nowadays, paramagnetic complexes of iridium(IV) are considered as promising building blocks for designing electronic and magnetic quantum materials.1,2 Earlier studies were essentially focused on oxoiridates with the principal structural fragment {IrO6}8–, and these compounds have been demonstrated to possess some intriguing physical properties, such as Mott spin–orbit insulators,3 superconductors,4−6 Weyl semimetallics,7−11 spin liquids and ices,12−15 and ferromagnets with anomalous Hall effect (AHE).16 With expectations that materials containing [IrF6]2– fragments also can exhibit specific physical properties,17 some efforts for the investigation of the electronic structures of (PPh4)2[IrF6]·2H2O, Zn(viz)4[IrF6], (PPh4)2[IrCl6],18 A2[IrF6] (A = Na, K, Rb, Cs), and Ba[IrF6]19 as well as the crystal structures of (PPh4)2[IrF6]·2H2O, Zn(viz)4[IrF6],18 Li2RhF6, K2IrF6,20 Rb2IrF6,21 Cs2IrF6,22 Ca[IrF6]·2H2O, Sr[IrF6]·2H2O, and Ba[IrF6]23 have been undertaken. The most convenient precursor for the synthesis of diverse hexafluoroiridates (IV) involving different cations is the salt Na2[IrF6]. As a rule, sodium salts of iridates are well soluble in water and therefore are handy starting compounds for ligand substitution reactions24 as well as cation metathesis.21−23 Although Na2[IrF6] has been known for a long time and its unit cell parameters have been reported (P321, a = 9.34 Å, c = 5.14 Å),25 followed by structural refinement in 2016 (P321, a = 9.32858(24) Å, c = 5.13417(19) Å),18 there are lacunas in the data on the structures of sodium fluoroiridates including the structure of the stable tetragonal phase Na2[IrF6]. Anhydrous sodium fluoroiridates (III) and (IV), as well as crystal hydrates commonly occurring on the crystallization of aqueous solutions, lack both powder and single-crystal structural data. These findings suggest that the studies on the preparation and structures of sodium fluoroiridates need to be revised.
2. Experimental Section
The PXRD experiments were examined on a DRON-RM4 diffractometer (Cu Kα source, graphite monochromator at the diffracted beam, room temperature, 2θ range 5–60°). The experimental data were processed with PowderCell program v.2.4.26 The data from the powder structural database PDF27 have been used as standards.
The single crystals were examined on an automated Bruker Nonius X8 APEX diffractometer (MoKα radiation 0.71073 Å, graphite monochromator, CCD detector) at 150(2)K. The reflection intensities were measured by φ scanning of narrow (0.5°) frames. Absorption is taken into account empirically using the SADABS program.28 Structures were solved by the direct methods of the difference Fourier synthesis and further refined by the full-matrix least-squares method using the SHELXTL package.29 Atomic thermal parameters for nonhydrogen atoms were refined anisotropically. The positions of hydrogen atoms for water molecules are not located.
A thermal analysis was performed on an “STA 449 F1 Jupiter” in a platinum crucible under a helium atmosphere in the temperature range 25–500 °C.
3. Results and Discussion
β-Na2[IrF6] (I) was prepared according to the literature method18 via the treatment of solid Na2[IrCl6] with gaseous fluorine under dynamic heating up to 300 °C in a flow reactor. Na2[IrCl6] was prepared from commercial “IrCl4·2H2O” in few steps (“The Gulidov Krasnoyarsk Non-Ferrous Metals Plant” Open Joint Stock Company, 51.9% iridium) by dissolution in concentrated hydrochloric acid, followed by the addition of the stoichiometric amount of NaCl and concentration to afford the dry salt. The attempted recrystallization of β-Na2[IrF6] (I) from aqueous solutions yielded single crystals of Na2[IrF6]·2H2O (II) but not those of (I). We expect that they can be prepared by recrystallization from anhydrous HF; however, such an experiment has not been carried out for technical reasons. Then, the powder of (I) has been examined with powder X-ray diffraction (Figure 1).
Figure 1.

Powder diffraction pattern of β-Na2[IrF6] (I) and its full-profile refinement.
The powder diffraction pattern of compound β-Na2[IrF6] (I) was simulated by the full-profile technique using the crystal data of an isostructural compound Na2[SiF6]30,31 (Figure 1), as, according to Babel,31β-Na2[IrF6] belongs to the structural type of Na2[SiF6] (rhombohedral cell, space group P321). The refinement of the unit cell parameters afforded the following values: sp. gr. P321, a = 9.332(4), c = 5.136(2) Å coinciding within uncertainty with the published data.18
It should be noted that the compound β-Na2[IrF6] is metastable and, similar to Na2[SnF6],32 it is converted to the tetragonal phase stable at room temperature with the progress of time. The diffraction pattern of a sample of β-Na2[IrF6] stored in a closed vial for two years exhibited reflections of the tetragonal phase α-Na2[IrF6]. The refinement of unit cell parameters gave the following data: sp. gr. P42/mnm, a = 5.005(2), c = 10.074(4) Å (Figure 2).
Figure 2.

Powder diffraction pattern of a mixture of α-Na2[IrF6] and β-Na2[IrF6] (I) and its full-profile refinement.
A comparison of α-Na2[IrF6] and β-Na2[IrF6] structures calculated by the Rietveld method using TOPAS v. 6.033 software is shown in Table 1. The structure of α-Na2[IrF6] is slightly denser due to the stronger interaction between sodium and fluorine.
Table 1. Comparison of α-Na2[IrF6] and β-Na2[IrF6].
| substance | bond distances in [IrF6]2– (Å) | bond distances in {NaF6} (Å) | calculated density (g/cm3) | ||
|---|---|---|---|---|---|
| α-Na2[IrF6], low temp. | Ir-F1 (4x) | 1.946 | Na-F1 (2x) | 2.248 | 4.63 |
| Ir-F2 (2x) | 1.940 | Na-F1 (2x) | 2.284 | ||
| Na-F2 (2x) | 2.354 | ||||
| average | 1.943 | average | 2.295 | ||
| β-Na2[IrF6], high temp. | Ir1-F1 (6x) | 1.940 | Na1-F2 (2x) | 2.204 | 4.52 |
| Ir2-F2 (3x) | 1.947 | Na1-F3 (2x) | 2.322 | ||
| Ir2-F2 (3x) | 1.933 | Na2-F1 (2x) | 2.407 | ||
| mean | 1.940 | mean | 2.311 | ||
The obtained single crystals of Na2[IrF6]·2H2O (II) were examined by single-crystal XRD. The crystal data of (II) is as follows: C2/m, a = 6.6327(4) Å, b = 10.0740(6) Å, c = 5.9283(5)Å, β = 122.3880(10)° (ICSD #1955565). The powder diffraction pattern of the bulk sample of (II) confirmed the analytical and phase purity of the product.
The interaction of 1.009 g of β-Na2[IrF6] (I) with 10 ml of H+-form of the cation exchanger KU-2 in 10 ml of water under stirring for 30 min (100 rpm) afforded a solution of H2[IrF6] (III). After the removal of the resin by filtration, the solution volume was added to 20 ml with the addition of water and the expected concentration C(Ir) was 0.143 M. The resultant solution was titrated with aqueous NaOH to give the proton content C(H+) of 0.289 M; the iridium concentration C(Ir) of 0.120 M was also determined by UV–vis spectroscopy.34 The determined concentration is less than the expected value, apparently, because of the partial sorption of iridium by the cation resin. Alkali titration gave only one equivalence point; hence, the solution is a strong acid in both steps. The reported behavior25 of (III) as a mixture of the strong acid in the first two steps and a weak acid in the third step has not been confirmed by us.
As reported in the literature,25 preparations of the crystals of H2[IrF6] (III) appeared to be unsuccessful. We expect that they can be prepared by the crystallization of IrF4 from anhydrous HF; however, such an experiment has not been carried out for technical reasons. The solution of (III) was used by us for the preparation of a series of salts; however, this contribution is related to only the synthesis of sodium salts, including the preparation from the acid (III).
The interaction of an aqueous solution of H2[IrF6] (III) with sodium chloride, followed by a low concentration in the air, gave large yellow crystals that were identified by single-crystal XRD (by the method described below) as Na3[IrF6]·2H2O (IV) (R-3, a = 7.5963(3), b = 7.5963(3), c = 9.8056(4) Å) (ICSD #1955580).
The powder diffraction pattern of the product (IV) prepared via interaction of (III) with a threefold excess of sodium chloride perfectly coincides with the pattern predicted from the single-crystal data (Figure 3).
Figure 3.

Powder diffraction pattern of Na3[IrF6]·2H2O (IV) and its comparison to the pattern predicted using the single-crystal data.
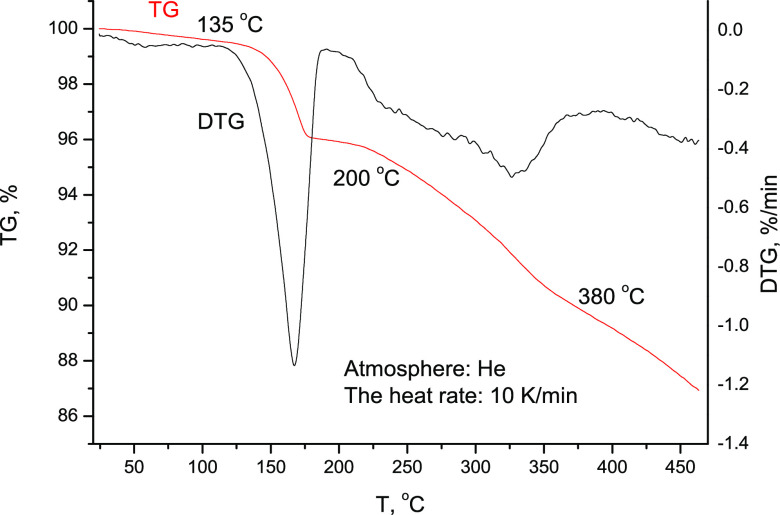
A gentle attempt to slowly remove water from Na3[IrF6]·2H2O (IV) by stepwise heating to 150 °C (step size of 10 °C) with thermal equilibration at 10–30 min resulted in a weight loss of ∼3% (theoretical water content 8.76%). According to the PXRD, the sample was an almost pure phase of (IV), so the partial removal of water did not result in an essential structural rearrangement of (IV). On heating up to 450 °C, the sample completely lost water within the temperature range of 135–400 °C, and underwent further slow partial decomposition (not more than 5%; Figure 4)
PXRD has demonstrated that the thermal decomposition of Na3[IrF6]·2H2O (IV) mostly afforded Na3[IrF6] (V). In addition to sodium hexafluoroiridate, the sample exhibited diffraction peaks of NaF and metallic Ir (Figure 5). The crystallographic data for the salt Ca3TeO6,35 isostructural to previously unknown Na3[IrF6] (V), were used for full-profile fitting of the unit cell parameters of compound (V): sp. gr. P21/n, a = 5.567(4), b = 5.778(4), c = 8.017(2) Å, and β = 90.41(2)°.
Figure 4.
TGA curves of water loss and decomposition of Na3[IrF6]·2H2O (IV) in the helium atmosphere.
Figure 5.

PXRD pattern of the products of thermal decomposition of Na3[IrF6]·2H2O (IV). The pattern of Na3[IrF6] was derived using the data for Ca3TeO6.
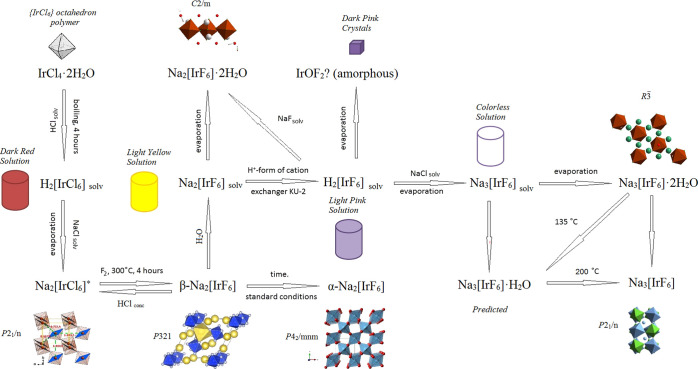
The common scheme of conversion in the Na(H)–Or–F(Cl) system is shown in Figure 6 (Na2[IrCl6], Na2[IrCl6]·2H2O, and Na2[IrCl6]·6H2O).36
Figure 6.
Common scheme of the conversion in the Na(H)–Ir–F(Cl) system.
4. Conclusions
The following salts have been synthesized and structurally characterized for the first time: Na2[IrF6]·2H2O (C2/m, a = 6.6327(4), b = 10.0740(6), c = 5.9283(5) Å, β = 122.3880(10)°) and Na3[IrF6]·2H2O (R-3, a = 7.5963(3), b = 7.5963(3), c = 9.8056(4) Å) by single-crystal XRD; unit cell parameters for β-Na2[IrF6] (P321, a = 9.332(4), c = 5.136(2) Å) and Na3[IrF6] (P21/n, a = 5.567(4), b = 5.778(4), c = 8.017(2) Å, β = 90.41(2)°) were determined from powder diffraction data. The crystal system and unit cell parameters of β-Na2[IrF6] coincide within experimental uncertainty with ours and the data of Hepworth,25 and newer results: sp. gr. P321, a = 9.332(4) Å, c = 5.136(2) Å. The unit cell parameters of the stable tetragonal phase α-Na2[IrF6] (P42/mnm, a = 5.005(2), c = 10.074(4) Å) were determined for the first time. H2[IrF6] was prepared in solution; and it was demonstrated to behave as a strong dibasic acid. The outline of the thermal decomposition curve suggests the presence of Na3[IrF6]·H2O. The common scheme of the conversion in Na(H)–Ir–F(Cl) was described.
Acknowledgments
This work was supported by the Russian Science Foundation, grant number 21-73-20203 (in the part of synthesis Na2[IrF6] and Na2[IrCl6]), and by the Ministry of Science and Higher Education of the Russian Federation, project number 121031700315-2 (in the part of chemistry investigation synthesized compounds in water and under thermal action).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c02722.
The authors declare no competing financial interest.
Supplementary Material
References
- Rau J. G.; Lee E. K.-H.; Kee H.-Y. Spin-Orbit Physics Giving Rise to Novel Phases in Correlated Systems: Iridates and Related Materials. Annu. Rev. Condens. Matter Phys. 2016, 7, 195–221. 10.1146/annurev-conmatphys-031115-011319. [DOI] [Google Scholar]
- Meyers D.; Cao Y.; Fabbris G.; Robinson N. J.; Hao L.; Frederick C.; Traynor N.; Yang J.; Lin J.; Upton M. H.; Casa D.; Kim J.-W.; Gog T.; Karapetrova E.; Choi Y.; Haskel D.; Ryan P. J.; Horak L.; Liu X.; Liu J.; Dean M. P. M. Magnetism in iridate heterostructures leveraged by structural distortions. Sci. Rep. 2019, 9, 4263 10.1038/s41598-019-39422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. J.; Ohsumi H.; Komesu T.; Sakai S.; Morita T.; Takagi H.; Arima T. Phase-sensitive observation of a spin-orbital Mott state in Sr2IrO4. Science 2009, 323, 1329–1332. 10.1126/science.1167106. [DOI] [PubMed] [Google Scholar]
- Kim Y. K.; Krupin O.; Denlinger J. D.; Bostwick A.; Rotenberg E.; Zhao Q.; Mitchell J. F.; Allen J. W.; Kim B. J. Fermi arcs in a doped pseudospin-1/2 Heisenberg antiferromagnet. Science 2014, 345, 187–190. 10.1126/science.1251151. [DOI] [PubMed] [Google Scholar]
- Kim Y. K.; Sung N. H.; Kim B. J.; et al. Observation of a d-wave gap in electron-doped Sr2IrO4. Nat. Phys. 2016, 12, 37–41. 10.1038/nphys3503. [DOI] [Google Scholar]
- Zhao L.; Torchinsky D. H.; Chu H.; Ivanov V.; Lifshitz R.; Flint R.; Qi T.; Cao G.; Hsieh D. Evidence of an odd-parity hidden order in a spin-orbit coupled correlated iridate. Nat. Phys. 2016, 12, 32–37. 10.1038/nphys3517. [DOI] [Google Scholar]
- Yang B.-J.; Kim Y. B. Topological insulators and metal-insulator transition in the pyrochlore iridates. Phys. Rev. B. 2010, 82, 085111 10.1103/PhysRevB.82.085111. [DOI] [Google Scholar]
- Wan X.; Turner A. M.; Vishwanath A.; Savrasov S. Y. Topological semimetal and Fermi-arc surface states in the electronic structure of pyrochlore iridates. Phys. Rev. B: Condens. Matter Mater. Phys. 2011, 83, 205101 10.1103/PhysRevB.83.205101. [DOI] [Google Scholar]
- Witczak-Krempa W.; Kim Y. B. Topological and magnetic phases of interacting electrons in the pyrochlore iridates. Phys. Rev. B 2012, 85, 045124 10.1103/PhysRevB.85.045124. [DOI] [Google Scholar]
- Donnerer C.; Pincini D.; Strempfer J.; Krisch M.; Prabhakaran D.; Boothroyd A. T.; McMorrow D. F.; et al. All-in–all-Out Magnetic Order and Propagating Spin Waves in Sm2Ir2O7. Phys. Rev. Lett. 2016, 117, 037201 10.1103/physrevlett.117.037201. [DOI] [PubMed] [Google Scholar]
- Fujioka J.; Yamada R.; Kawamura M.; Sakai S.; Hirayama M.; Arita R.; Okawa T.; Hashizume D.; Hoshino M.; Tokura Y. Strong-correlation induced high-mobility electrons in Dirac semimetal of perovskite oxide. Nat. Commun. 2019, 10, 362 10.1038/s41467-018-08149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida Y.; Nakatsuji S.; Onoda S.; Tayama T.; Sakakibara T. Time-reversal symmetry breaking and spontaneous Hall effect without magnetic dipole order. Nature 2010, 463, 210–213. 10.1038/nature08680. [DOI] [PubMed] [Google Scholar]
- Modic K. A.; Smidt T. E.; Kimchi I.; Breznay N. P.; Biffin Al.; Choi S.; Johnson R. D.; Coldea R.; Watkins-Curry P.; McCandless G. T.; Chan J. Y.; Gandara F.; Islam Z.; Vishwanath A.; Shekhter A.; McDonald R. D.; Analytis J. G. Realization of a three-dimensional spin-anisotropic harmonic honeycomb iridate. Nat. Commun. 2014, 5, 4203 10.1038/ncomms5203. [DOI] [PubMed] [Google Scholar]
- Nishimoto S.; Katukuri V. M.; Yushankhai V.; Stoll H.; Rößler U. K.; Hozoi L.; Rousochatzakis I.; Van den Brink J. Strongly frustrated triangular spin lattice emerging from triplet dimer formation in honeycomb Li2IrO3. Nat. Commun. 2016, 7, 10273 10.1038/ncomms10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. S.; Catuneanu A.; Sørensen E. S.; Kee H.-Y. Theory of the field-revealed Kitaev spin liquid. Nat. Commun. 2019, 10, 2470 10.1038/s41467-019-10405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowal S.; Satpathy S. Electric field tuning of the anomalous Hall effect at oxide interfaces. npj Comput. Mater. 2019, 5, 61 10.1038/s41524-019-0198-8. [DOI] [Google Scholar]
- Birol S.; Haule K. Jeff 1/4 1/2 Mott-insulating state in Rh and Ir fluorides. Phys. Rev. Lett. 2015, 114, 096403 10.1103/PhysRevLett.114.096403. [DOI] [PubMed] [Google Scholar]
- Pedersen K. S.; Bendix J.; Tressaud A.; Durand E.; Weihe H.; Salman Z.; Morsing T. J.; Woodruff D. N.; Lan Y.; Wernsdorfer W.; Mathoniere C.; Piligkos S.; Klokishner S. I.; Ostrovsky S.; Ollefs K.; Wilhelm F.; Rogalev A.; Clerac R. Iridates from the molecular side. Nat. Commun. 2016, 7, 12195 10.1038/ncomms12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M.; Retegan M.; Giacobbe C.; Fumagalli R.; Efimenko A.; Kulka T.; Wohlfeld K.; Gubanov A. I.; Sala M. M. Possibility to realize spin-orbit-induced correlated physics in iridium fluorides. Phys. Rev. 2017, B 95, 235161 10.1103/physrevb.95.235161. [DOI] [Google Scholar]
- Fitz H.; Müller B. G.; Graudejus O.; Bartlett N. Einkristalluntersuchungen an LiMF6 (M = Rh, Ir), Li2RhF6 und K2IrF6. Anorg. Allg. Chem. 2020, 628, 133–137. . [DOI] [Google Scholar]
- Smolentsev A. I.; Naumov D. Y.; Gubanov A. I.; Danilenko A. M. Rubidium hexafluoridoiridate (IV). Acta Crystallogr., Sect. E: Struct. Rep. Online 2007, 63, i200. 10.1107/S1600536807059995. [DOI] [Google Scholar]
- Smolentsev A. I.; Naumov D. Y.; Gubanov A. I.; Danilenko A. M. Caesium hexafluoridoiridate (IV). Acta Crystallogr., Sect. E: Struct. Rep. Online 2007, 63, i201. 10.1107/S160053680706000X. [DOI] [Google Scholar]
- Smolensev A. I.; Gubanov A. I.; Danilenko A. M. Three hexafloridoiridates(IV), Ca[IrF6]·2H2O, Sr[IrF6]•2H2O and Ba[IrF6]. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2007, 63, i99–i101. 10.1107/s0108270107044046. [DOI] [PubMed] [Google Scholar]
- Isakova V. G.; Baidina I. A.; Morozova N. B.; Igumenov I. K. g-Halogenated iridium (III) acetylacetonates. Polyhedron 2000, 19, 1097–1103. 10.1016/S0277-5387(00)00358-2. [DOI] [Google Scholar]
- Hepworth M. A.; Robison P. L.; Westland G. J. 119. Complex Fluorides of Quadrivalent Osmium and Iridium and Corresponding Free Acids. J. Chem. Soc. 1958, 611 10.1039/jr9580000611. [DOI] [Google Scholar]
- Kraus W.; Nolze G.. PowderCell 2.4, Program for the Representation and Manipulation of Crystal Structures and Calculation of the Resulting X-ray Powder Patterns. Federal Institute for Materials Research and Testing: Berlin, Germany, 2000.
- ICDD PDF-2 Release 2014. International Centre for Diffraction Data: Swarthmore, PA, USA, 2014.
- Bruker AXS Inc. APEX (Version 1.08), SAINT (Version 7.03) and SADABS (Version 2.11). Bruker Advanced X-Ray Solutions: Madison, Wisconsin, USA, 2004.
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin B.; Forrester J. D.; Templeton D. H. The crystal structure of sodium fluorosilicate. Acta Cryst. 1964, 17, 1408–1412. 10.1107/S0365110X64003516. [DOI] [Google Scholar]
- Babel D.Structural Chemistry of Octahedral Fluorocomplexes of the Transition Elements. In Structure and Bonding; Springer: Berlin, Heidelberg, 1967; Vol. 3, pp 1–87. [Google Scholar]
- Grannec J.; Fournes L.; Lagassie P. X-ray and Mossbauer Evidence for a High Temperature Form of Na2SnF6. Mater. Res. Bull. 1990, 25, 1035–1041. 10.1016/0025-5408(90)90011-P. [DOI] [Google Scholar]
- Coelho A. A.. TOPAS-Academic V.6.0 General Profile and Structure Analysis Software for Powder Diffraction Data. http://www.topas-academic.net/, 2016.
- Zolotov Y. A.; Varshal G. M.; Ivanov V. M.. Analiticheskaya himiya metallov platinovoy gruppy [Analytical chemistry of Platinum metals]; Komkniga Publ: Moscow, 2005. [Google Scholar]
- Hottentot D.; Loopstra B. O. The structure of calcium orthotellurate. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 1981, B37, 220–222. 10.1107/S0567740881002550. [DOI] [Google Scholar]
- Bao S.-S.; Wang D.; Xuang X.-D.; Etter M.; Cai Z.-S.; Wan X.; Dinnebier R. E.; Zheng L.-M. Na2IrIVCl6: Spin–Orbital-Induced Semiconductor Showing Hydration-Dependent Structural and Magnetic Variations. Inorg. Chem. 2018, 57, 13252–13258. 10.1021/acs.inorgchem.8b01753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.